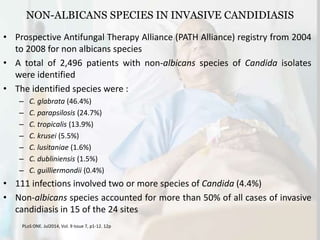
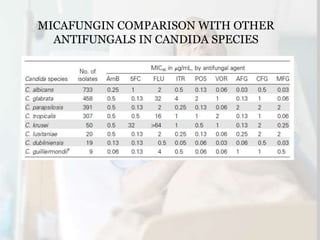
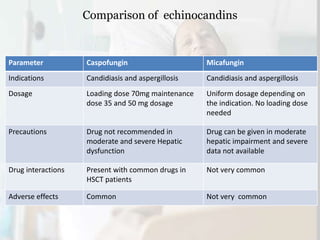
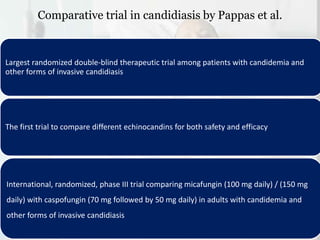
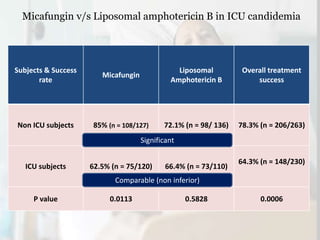
This document summarizes the antifungal drug micafungin and its use for invasive candidiasis. Micafungin is an echinocandin that inhibits fungal cell wall synthesis. It demonstrates efficacy comparable to other antifungals like caspofungin and liposomal amphotericin B for treating invasive candidiasis including candidemia. Micafungin has advantages over other drugs like fewer drug interactions, safety in hepatic impairment, and convenient once daily dosing without loading doses. It is effective against common Candida species including fluconazole and azole resistant strains.