Embed presentation
Downloaded 18 times

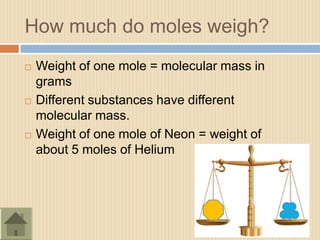
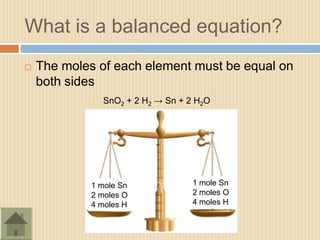
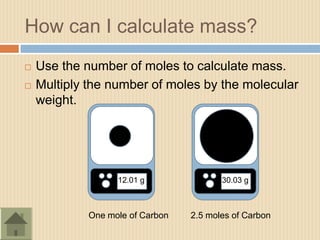

The document discusses the mole concept in chemistry. It defines a mole as 6.022x1023 particles of an element or compound. It explains that the mass of one mole of a substance is equal to its molecular mass in grams. Different substances have different molecular masses, so one mole of one element will weigh more or less than one mole of another element. The document also discusses how balanced chemical equations show equal numbers of moles on each side and how moles can be used to calculate mass by multiplying the number of moles by the molecular weight.





