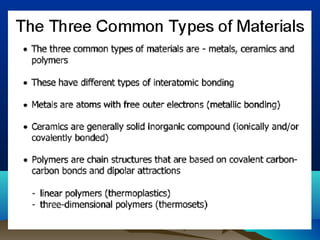
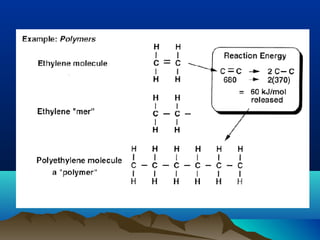
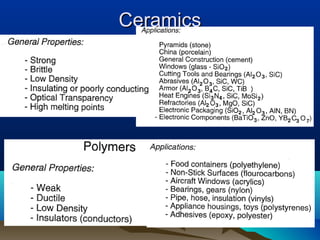
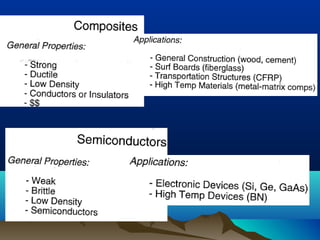
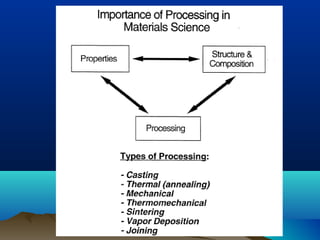
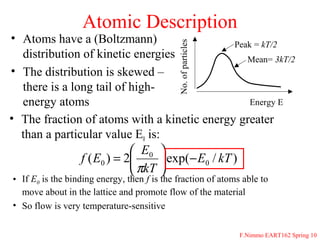
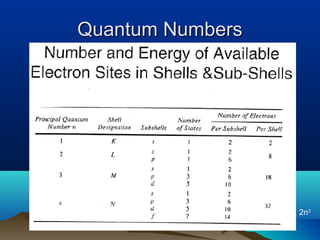
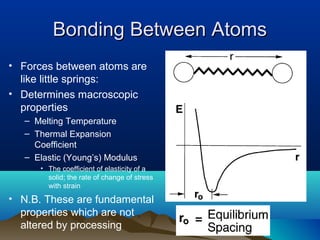
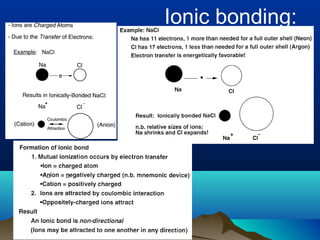
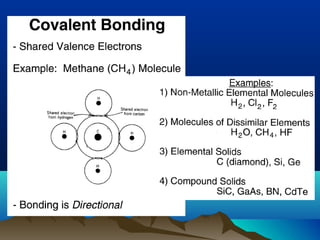
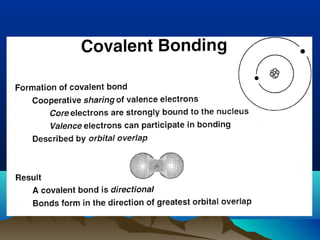
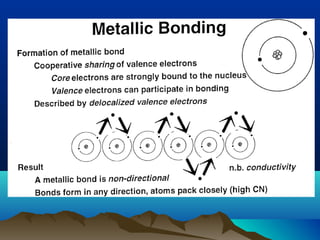
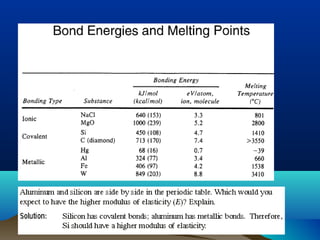
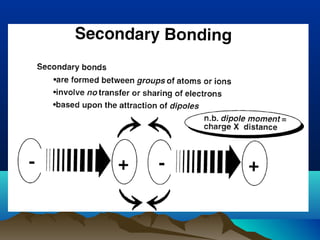
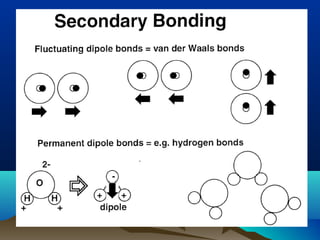
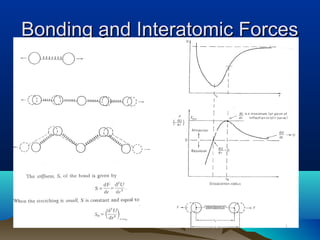
The document provides information on a materials science course taught by Danyuo Yiporo. It includes the instructor's contact information, rules and regulations, teaching strategies, course assessment details, course content outline, and recommended textbooks. The course will use lectures, tutorials, assignments, quizzes, tests and exams to teach topics like atomic structure, crystals, alloys, properties of materials, and different classes of materials.