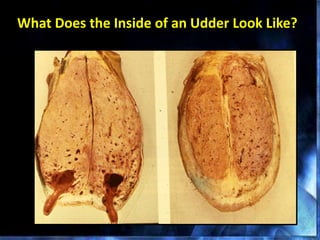
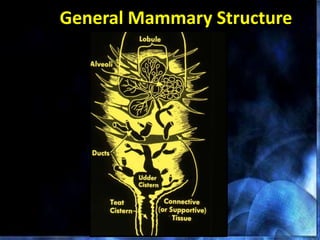
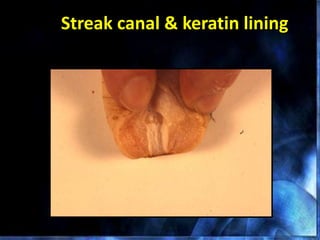
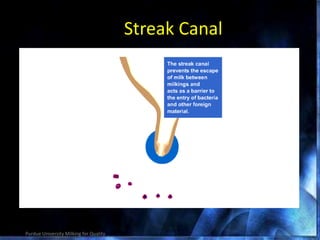
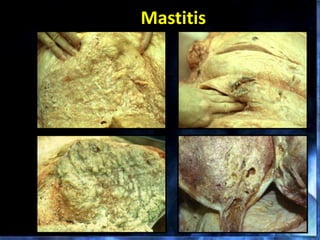
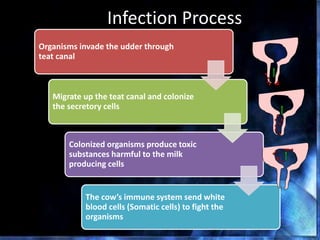
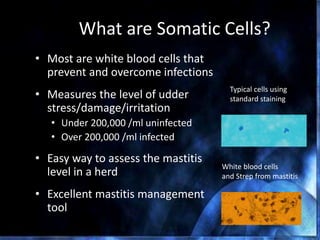
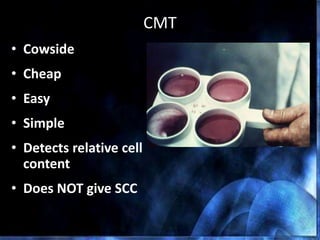
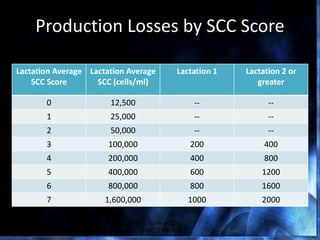
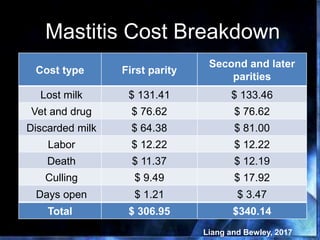
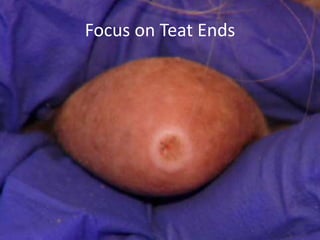
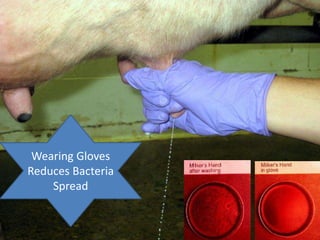
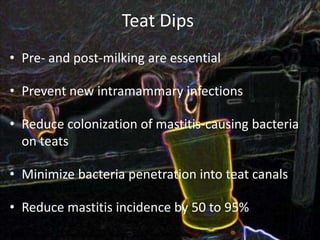
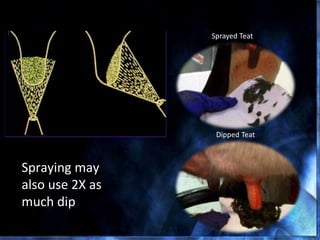
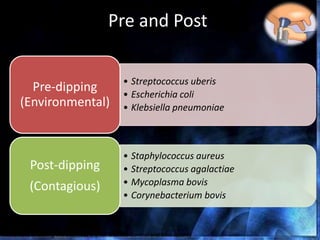
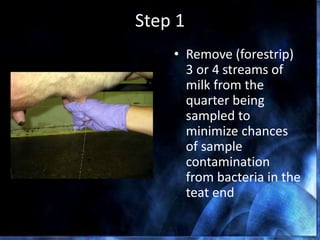
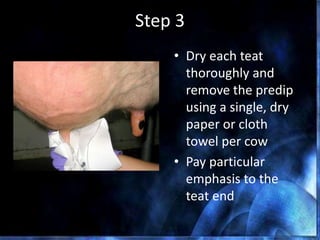
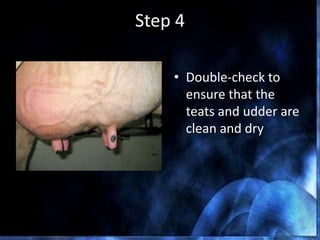
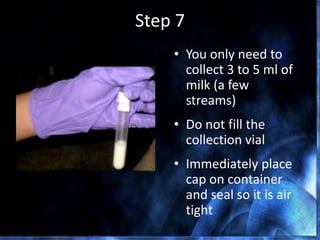
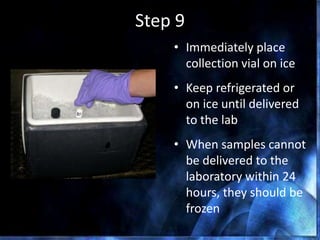
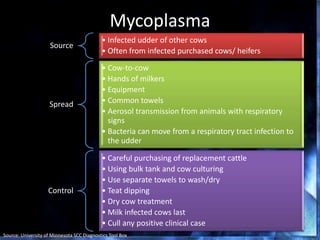
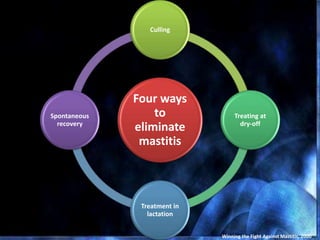
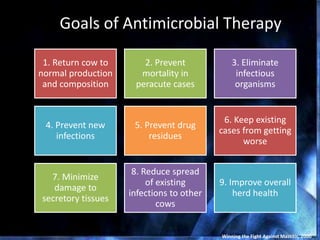
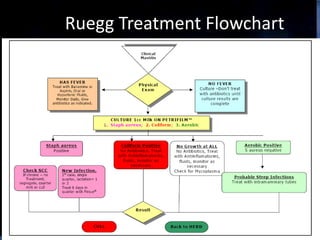
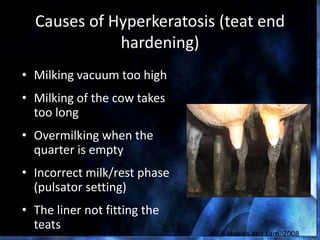
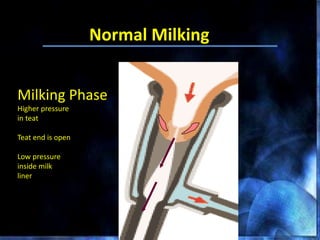
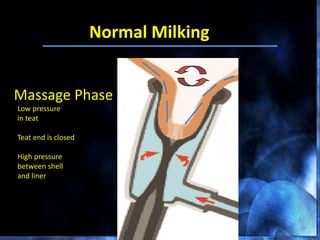
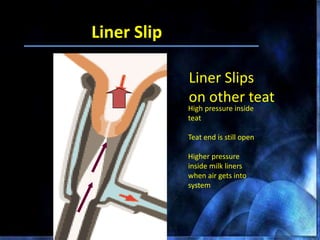
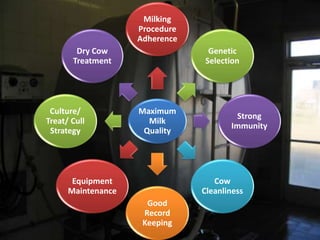
This document provides information on mastitis management and prevention. It discusses the structure of the mammary gland and defines mastitis as an inflammation of the mammary gland typically caused by bacteria. It notes that mastitis is the most costly disease in the dairy industry. It describes clinical versus subclinical mastitis and explains that subclinical mastitis has the greatest financial losses. It provides details on mastitis prevention including hygiene, environmental management, milking procedures, dry cow treatment, vaccination, and culture testing to identify causative bacteria and guide treatment decisions.