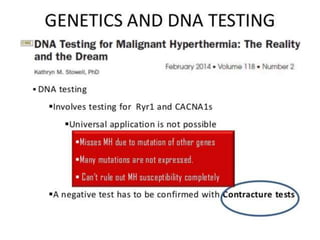
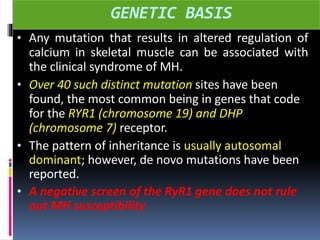
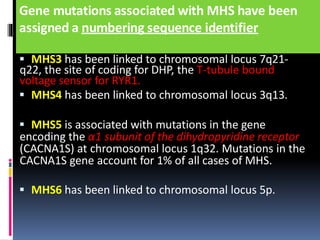
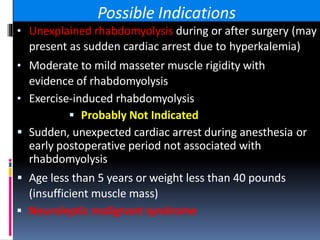
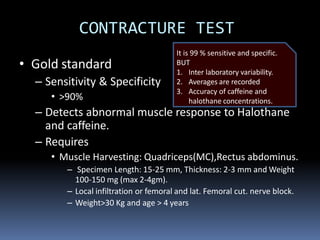
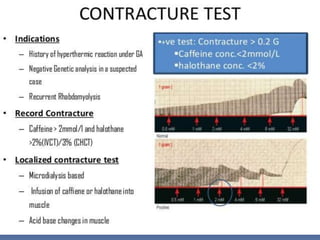
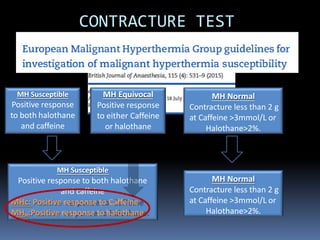
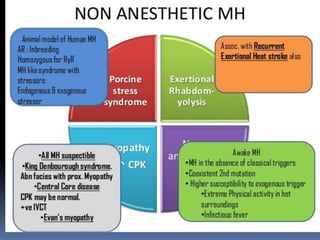
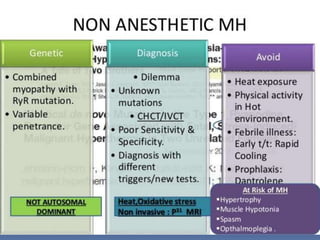
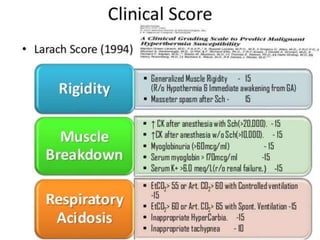
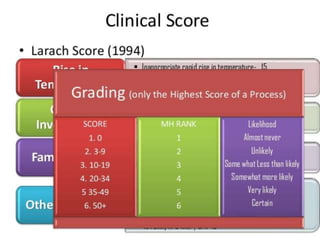
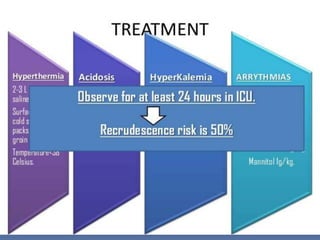
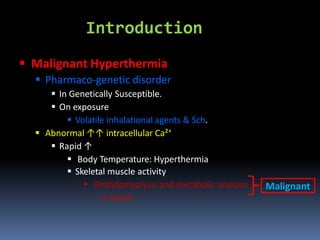
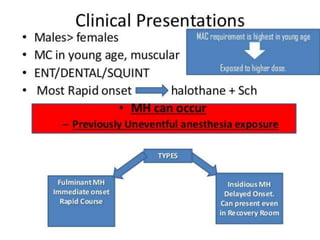
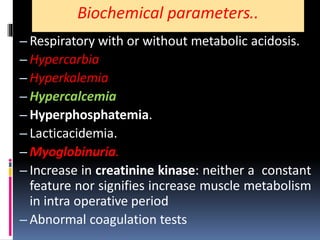
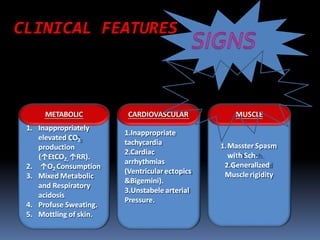
Malignant hyperthermia is a rare, potentially life-threatening condition triggered by certain anesthetic agents. It causes a hypermetabolic response in skeletal muscle that can rapidly increase body temperature, heart rate, and carbon dioxide levels. Left untreated, it can lead to muscle breakdown, acidosis, and death. The document discusses the definition, causes, signs and symptoms, diagnosis through muscle biopsy testing, genetic basis involving mutations of the ryanodine receptor gene, and treatment of malignant hyperthermia.






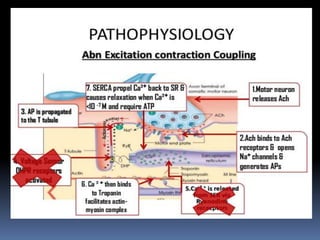
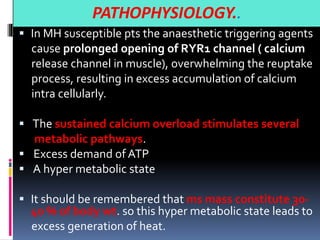
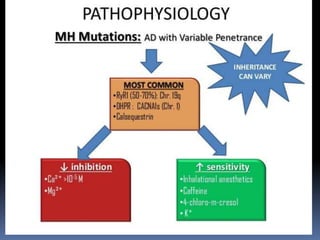
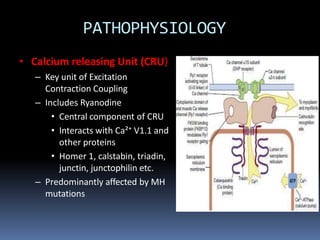
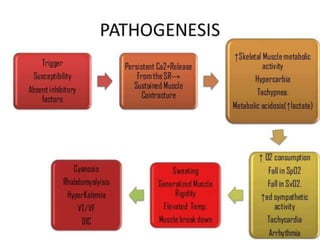
![PATHOPHYSIOLOGY..
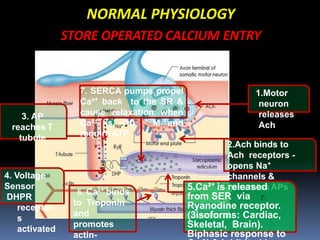
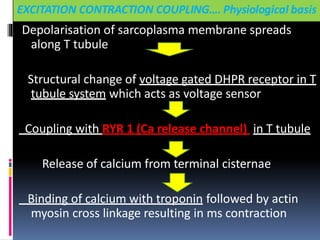
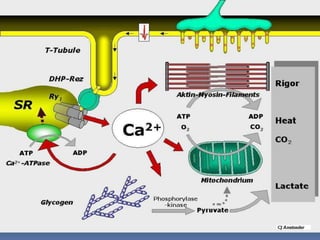
The sustained increase in [Ca2+]i leads to muscle
contraction without relaxation, i.e. spasm, which, if
prolonged, develops into severe contracture.
The muscle contracture greatly increases the extra
vascular resistance to muscle perfusion resulting in
ischemia initially locally and eventually systemically
The excess oxygen need due to demand for
production of extra ATP relative to diminished
perfusion due to ischemia lead to metabolic
exhaustion, muscle oedema and ultimately result in
muscle breakdown.](https://image.slidesharecdn.com/malignanthyperthermiafinal-final-190409162925/85/Malignanthyperthermiafinal-and-anaesthetic-consideration-19-320.jpg)