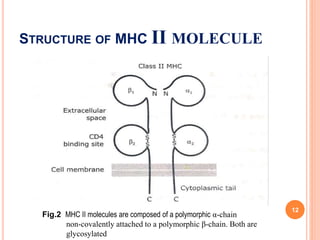
The presentation covers the Major Histocompatibility Complex (MHC), which is crucial for immune response through its role in antigen presentation. MHC molecules are classified into three classes: Class I, Class II, and Class III, each with distinct structures and functions in immune recognition. MHC molecules bind peptide fragments and present them to T cells, facilitating the immune system's ability to distinguish between self and non-self.