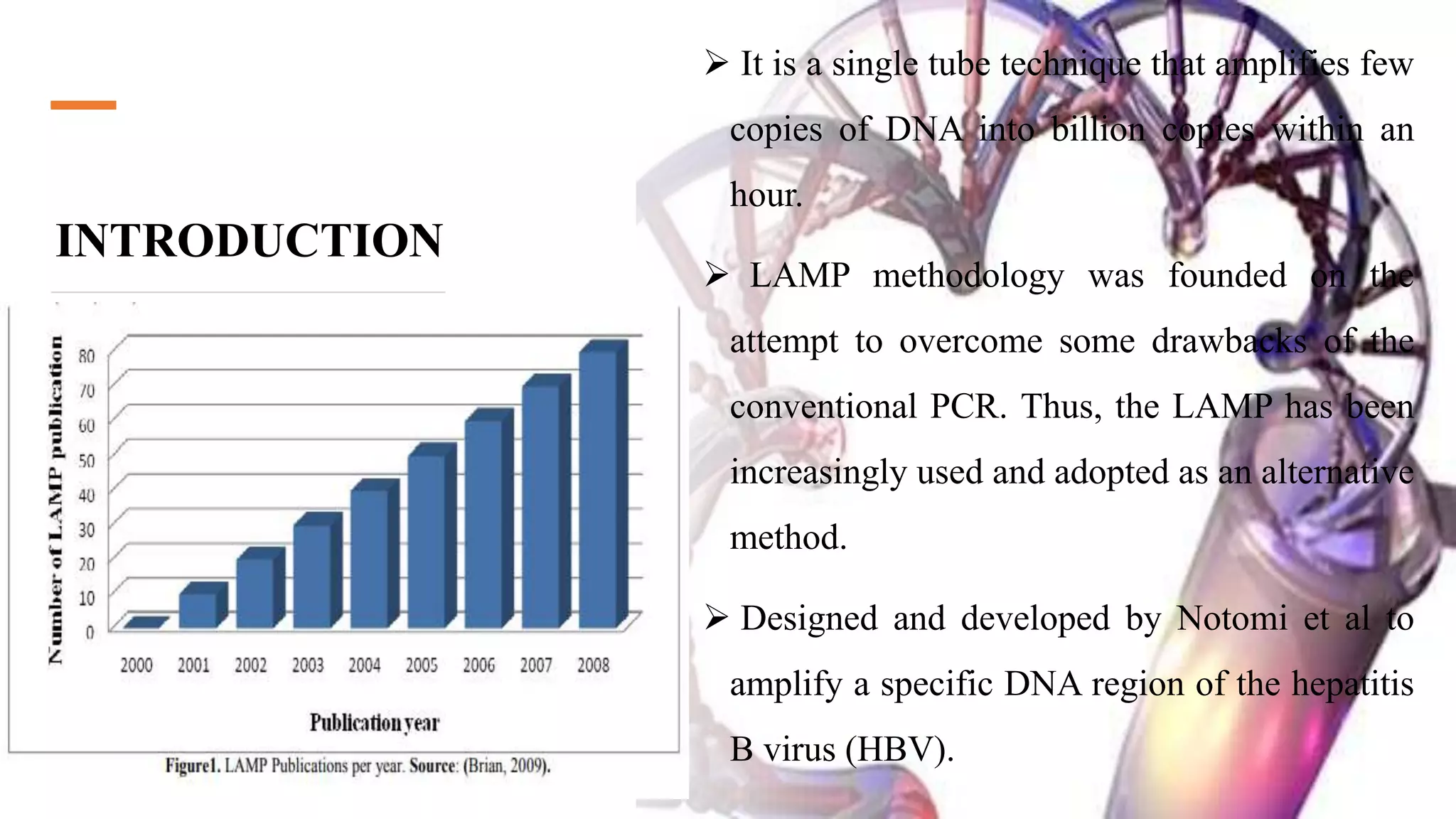
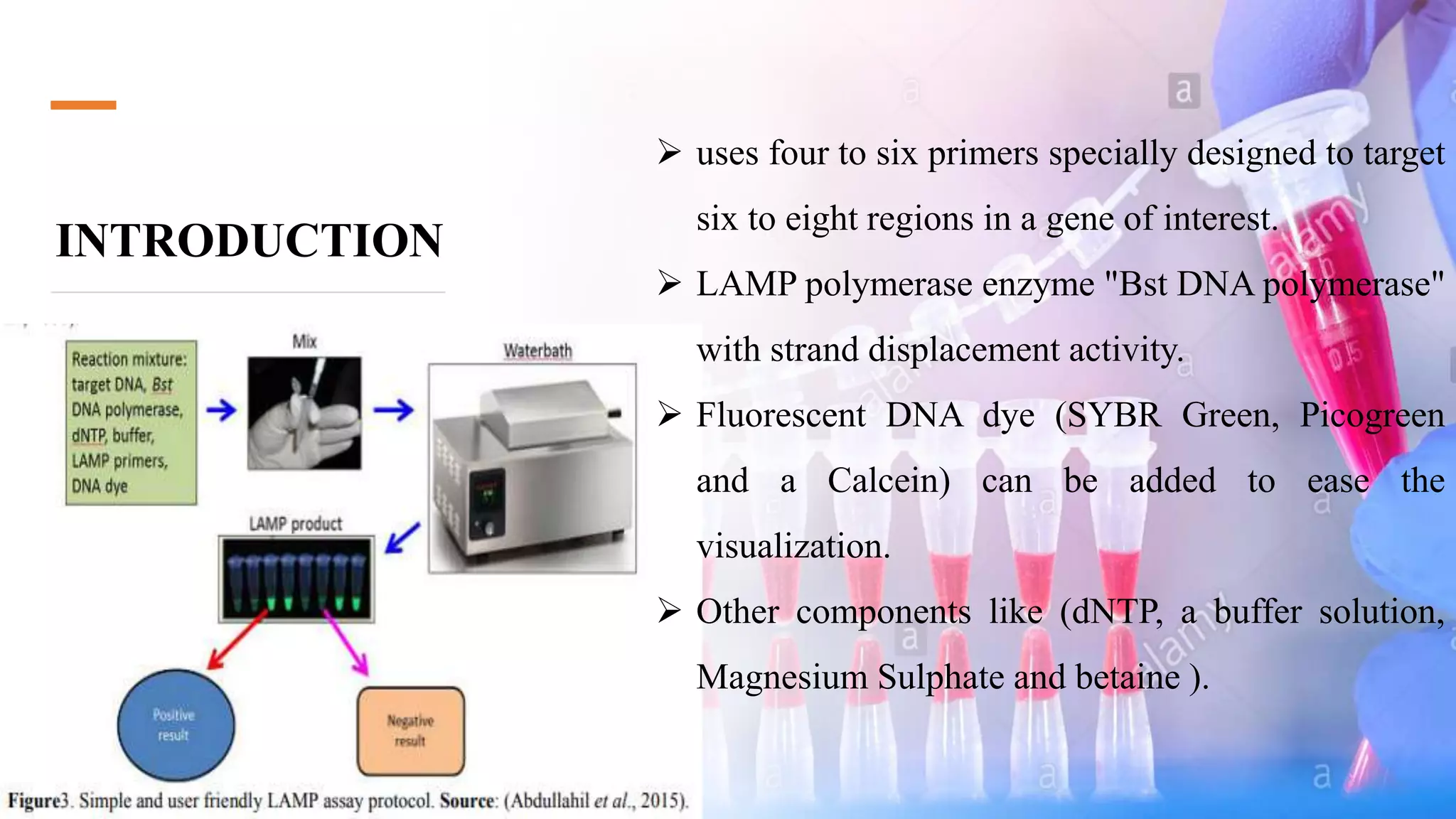
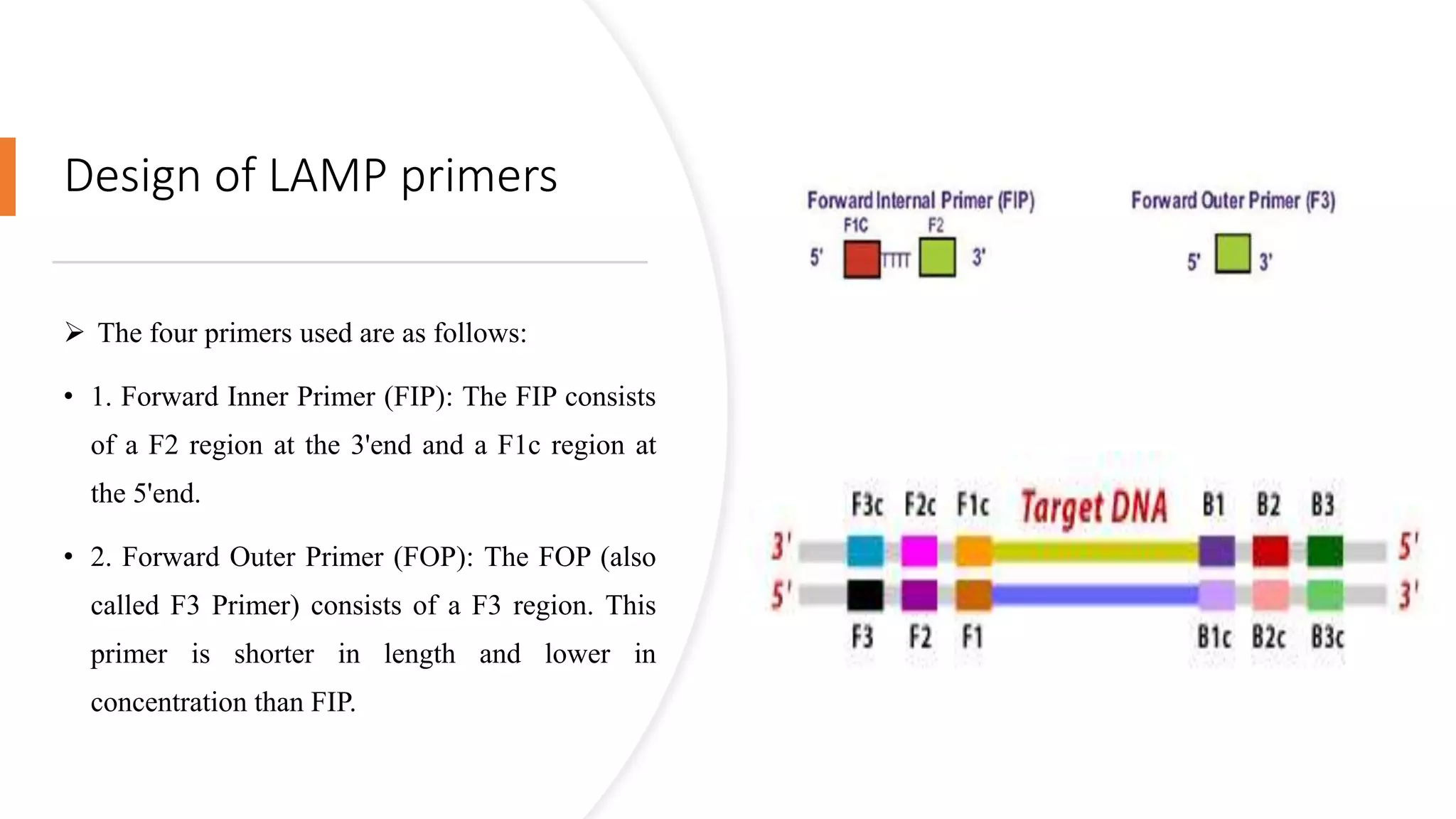
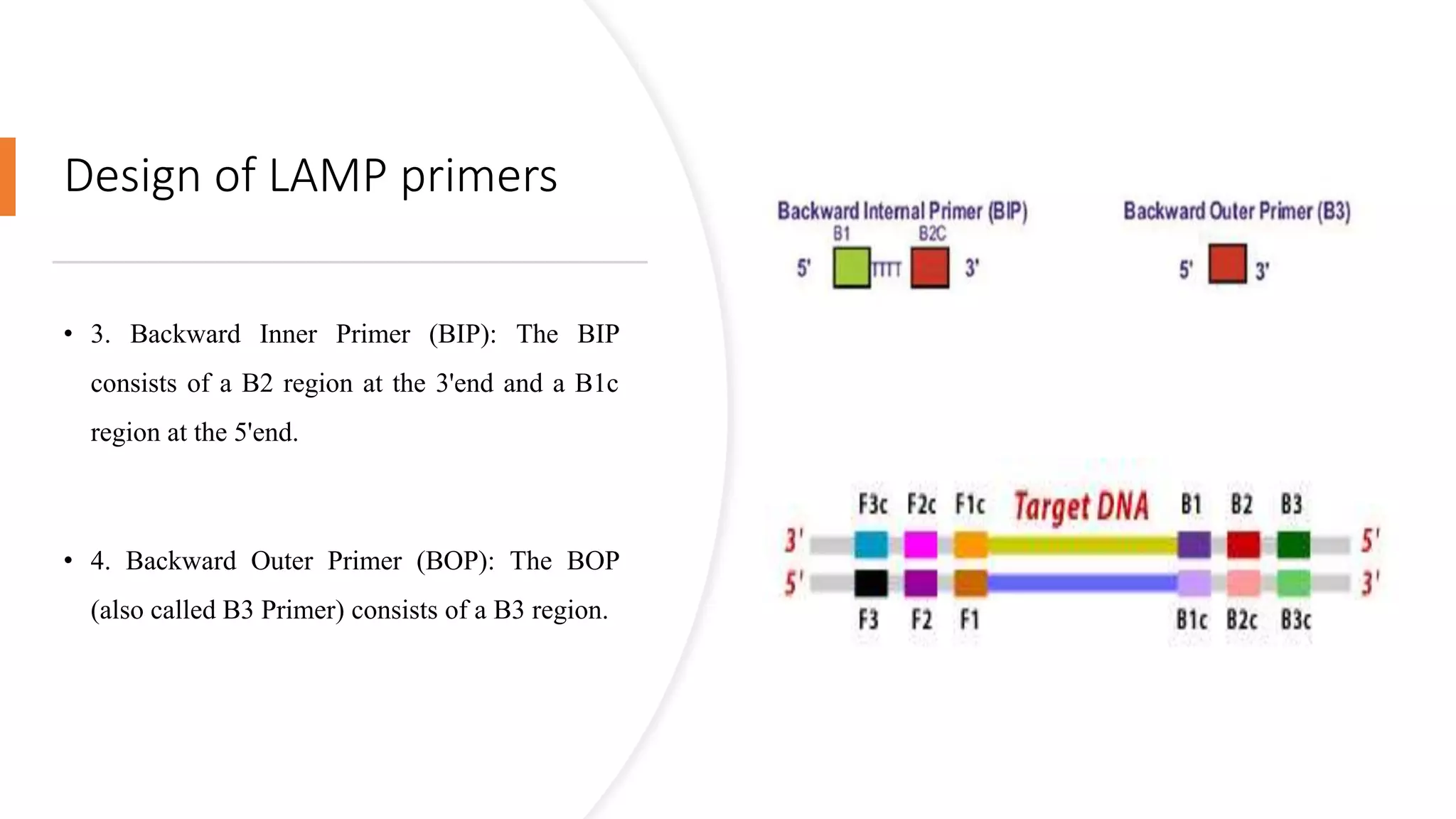
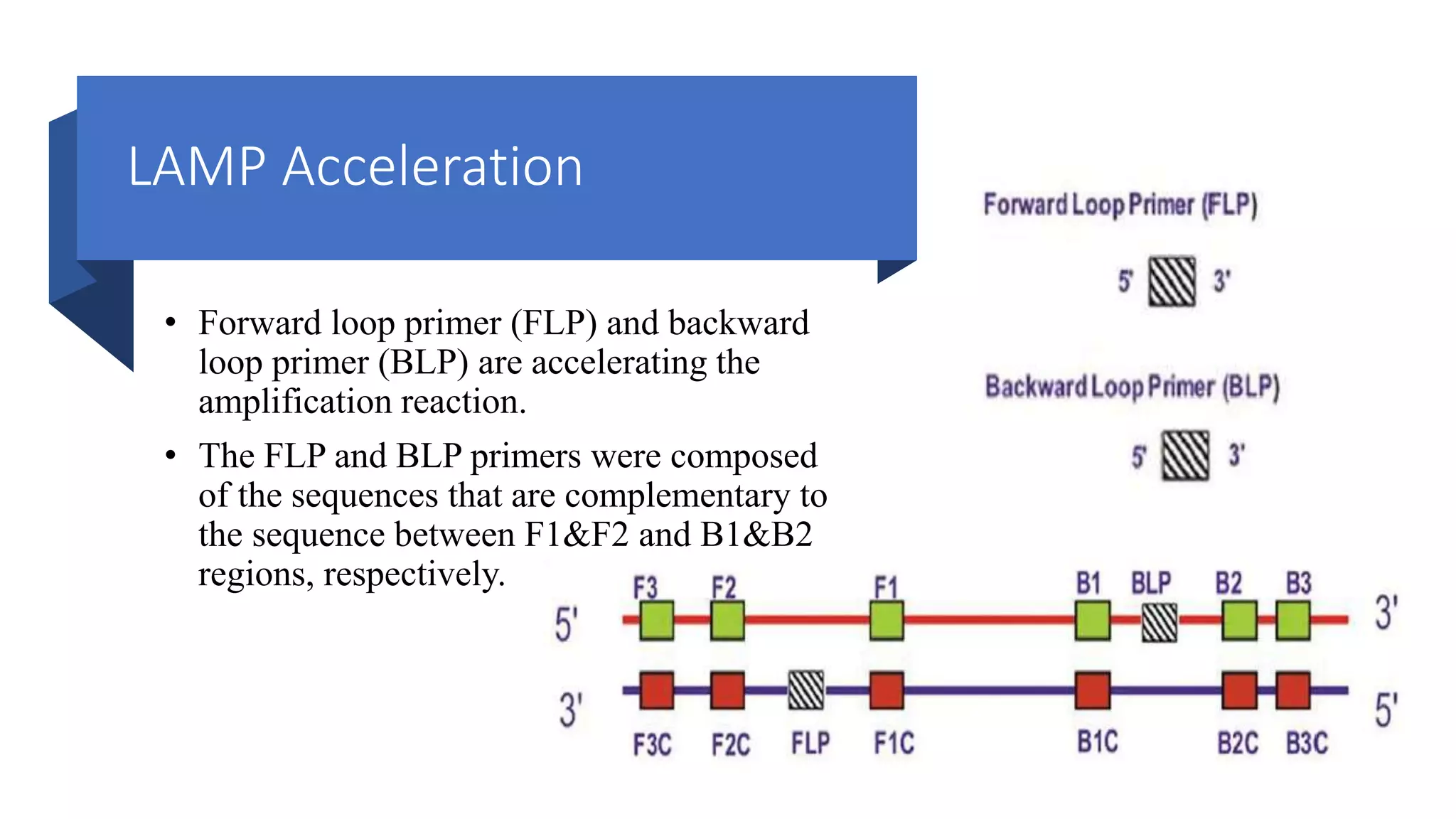
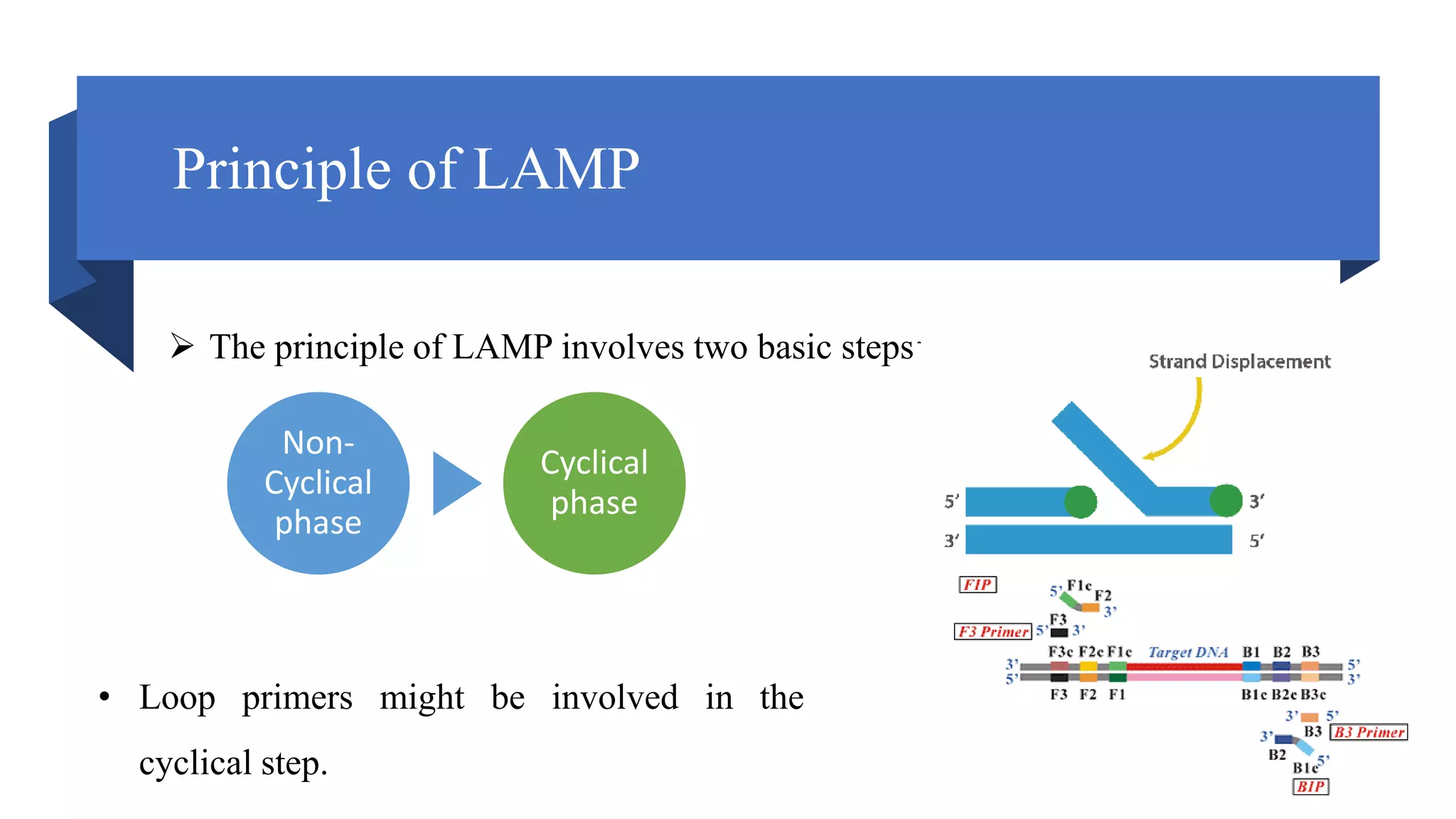
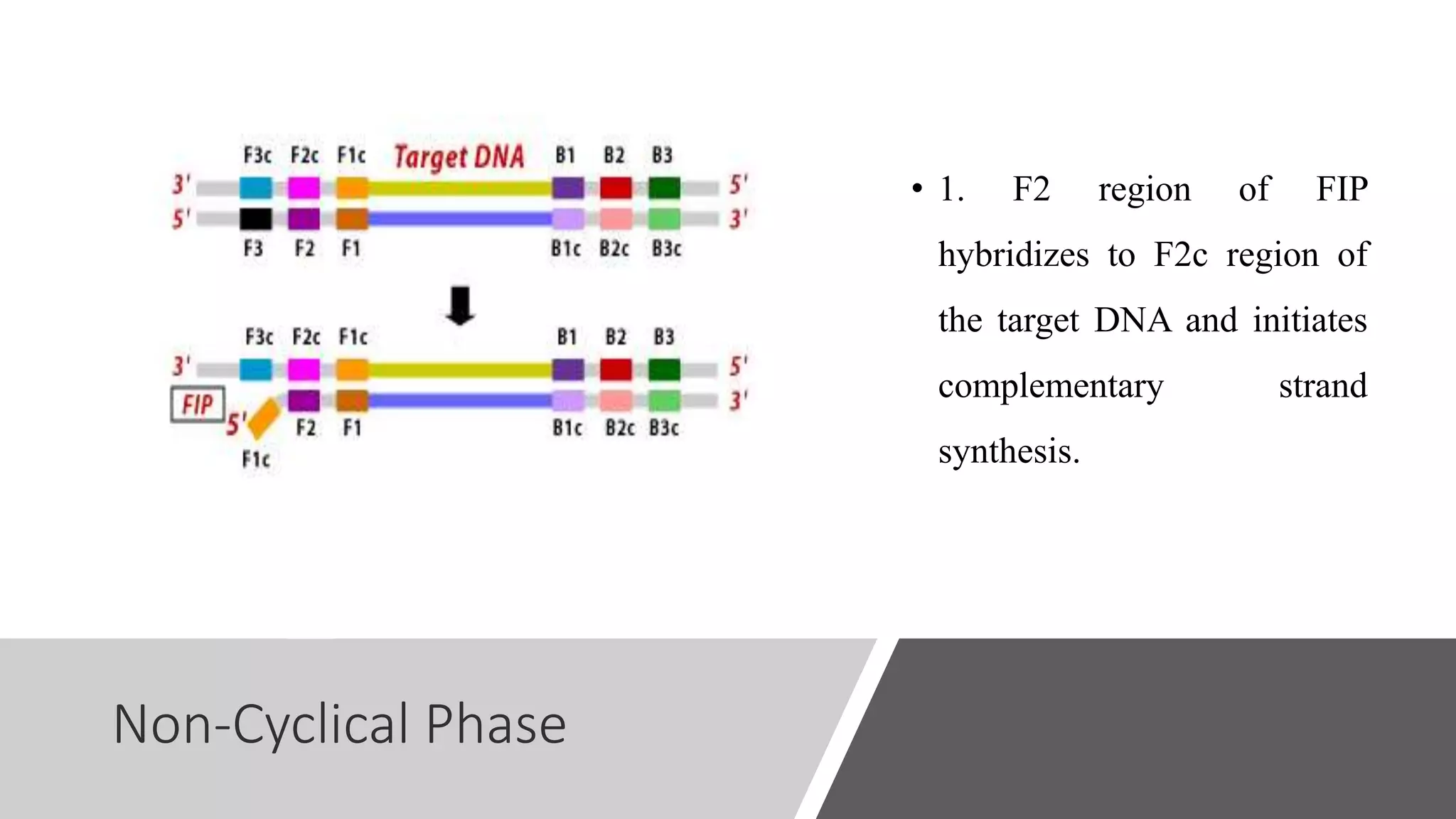
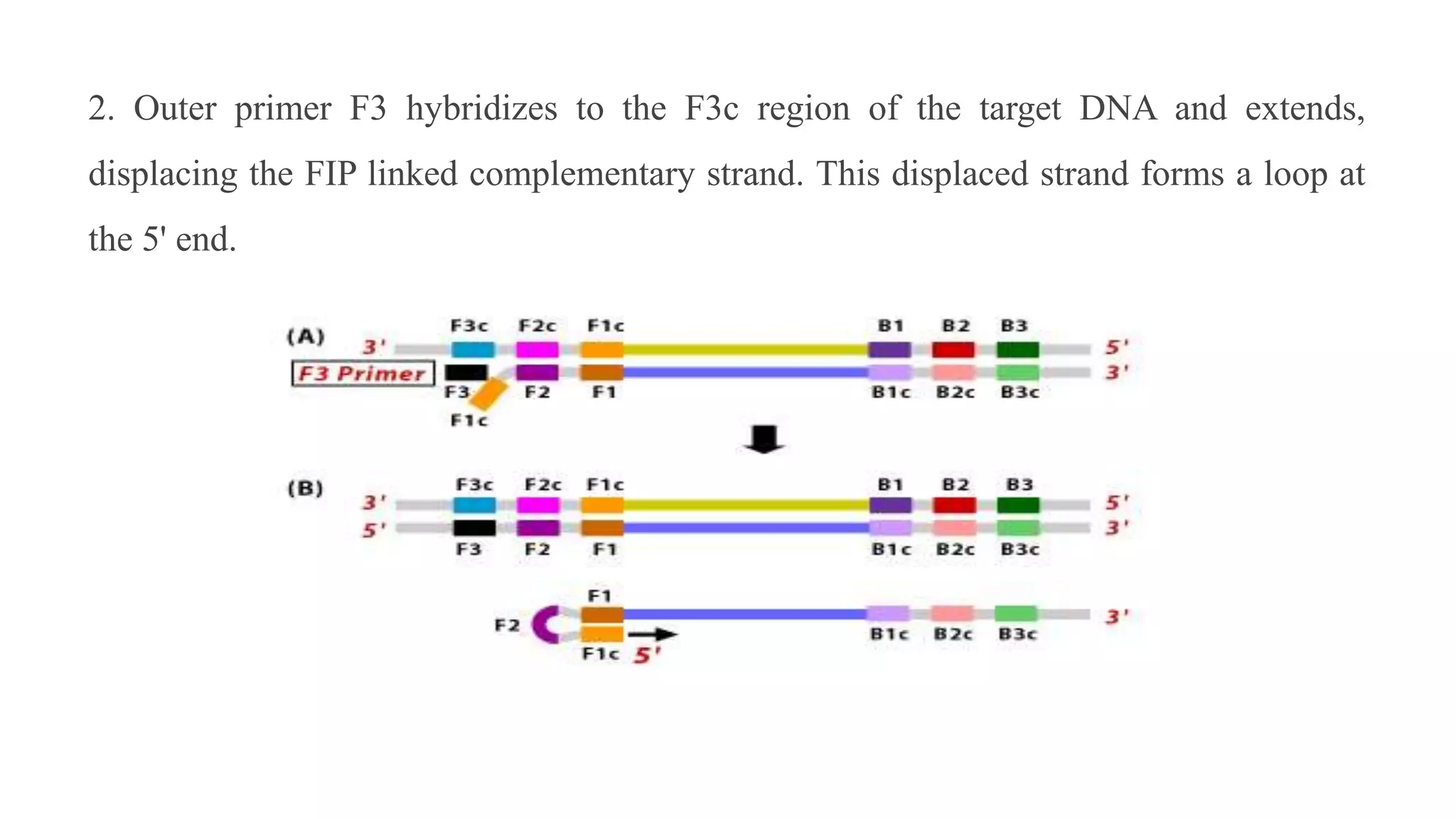
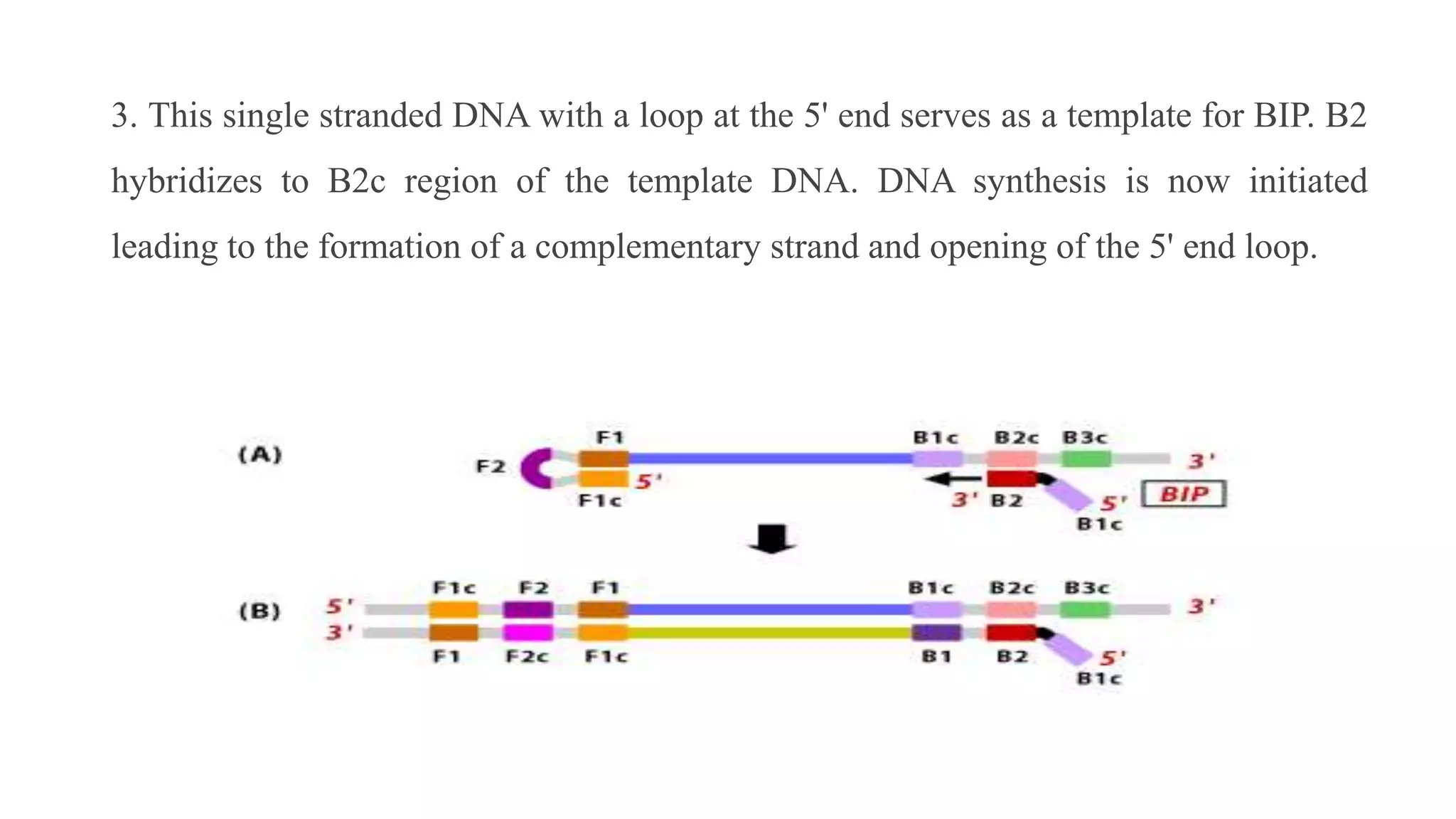
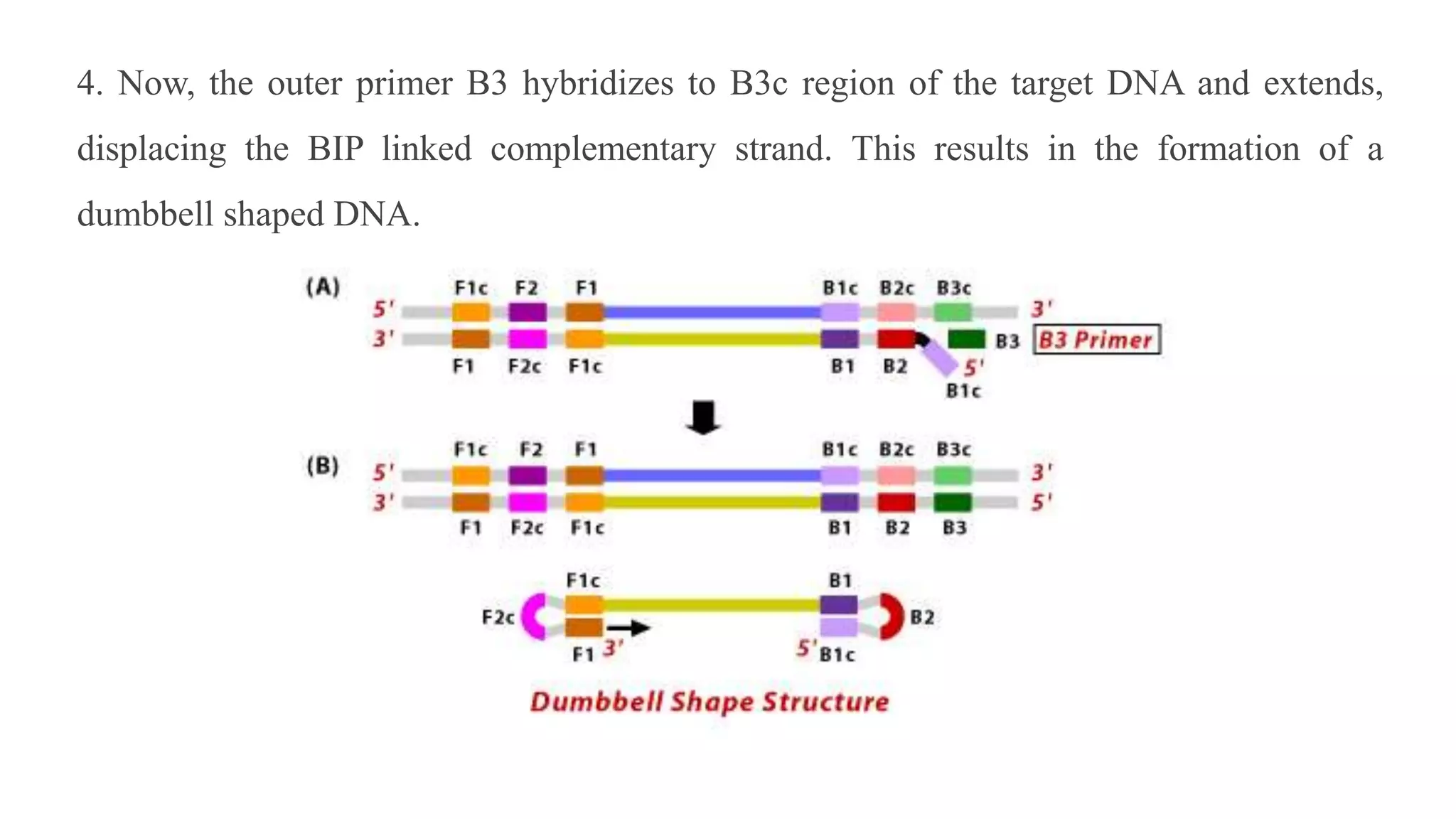
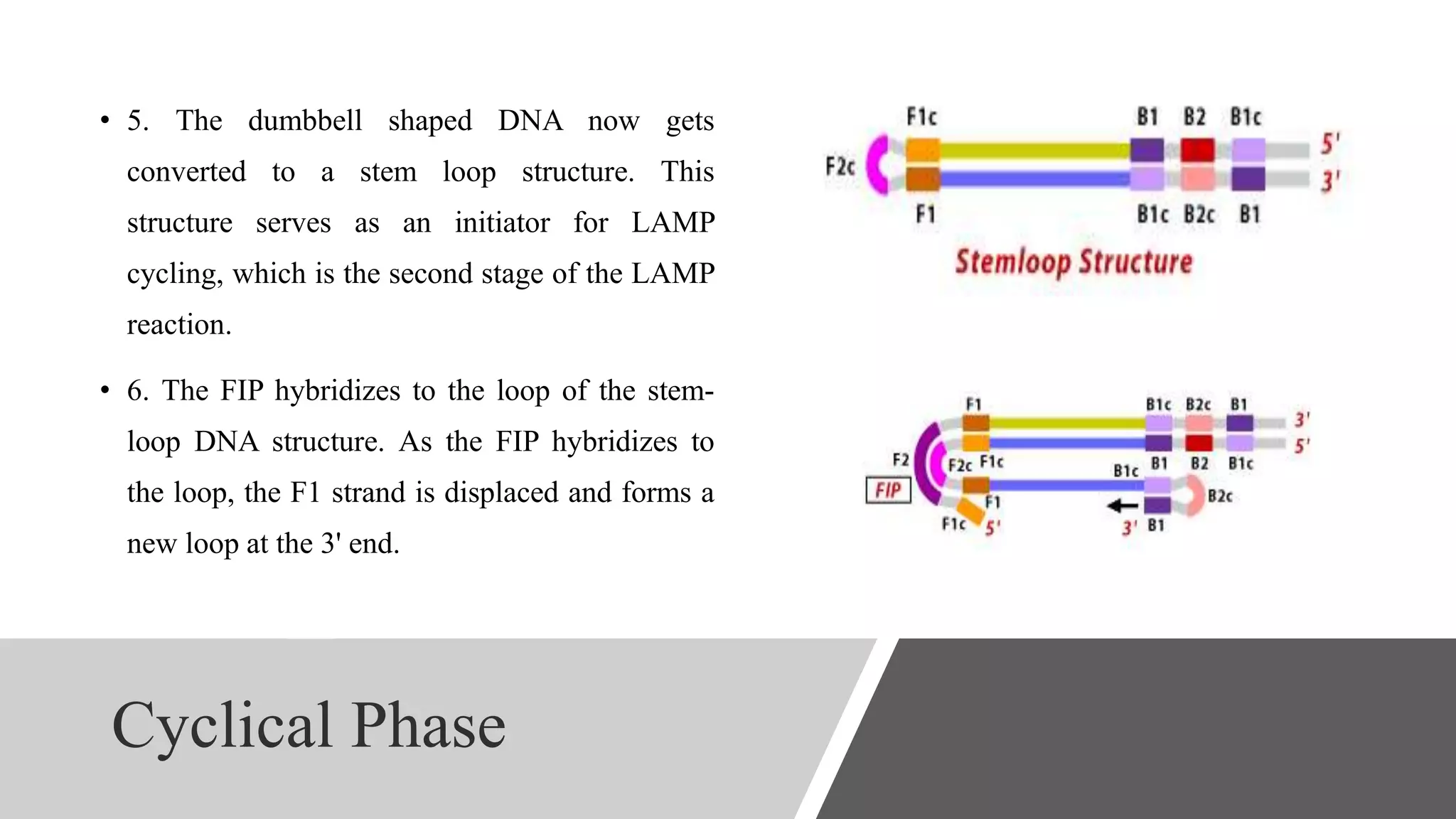
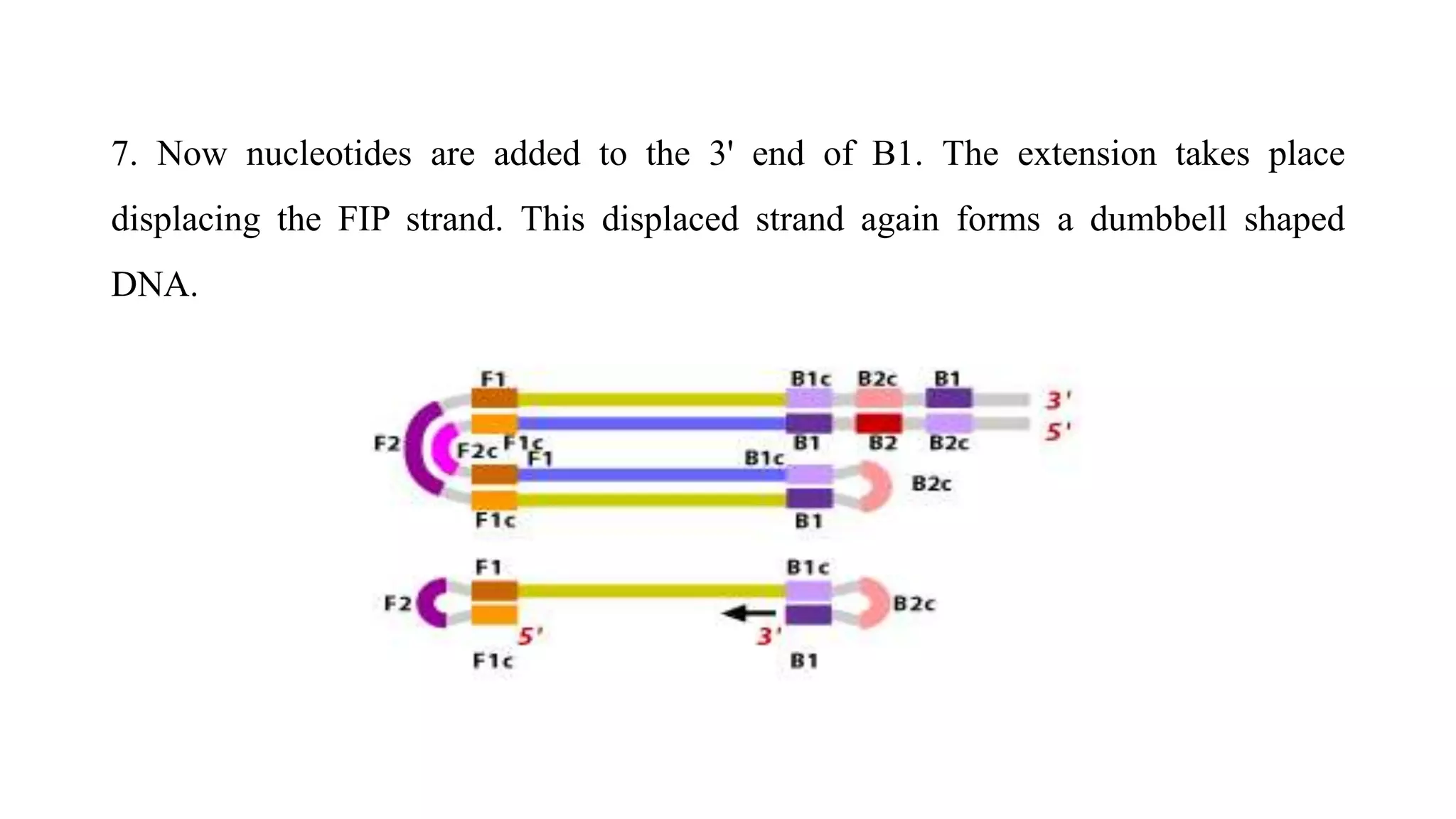
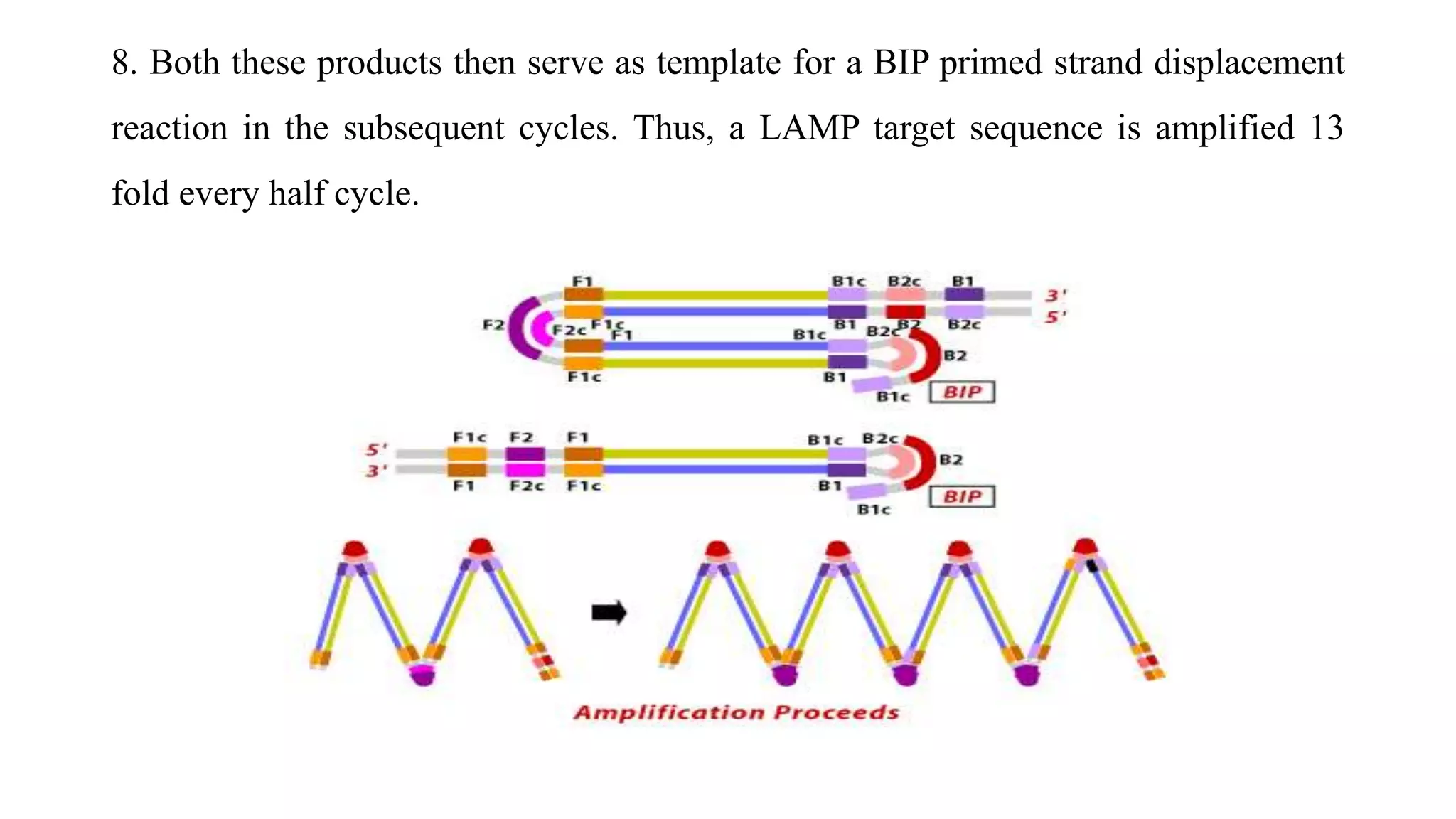
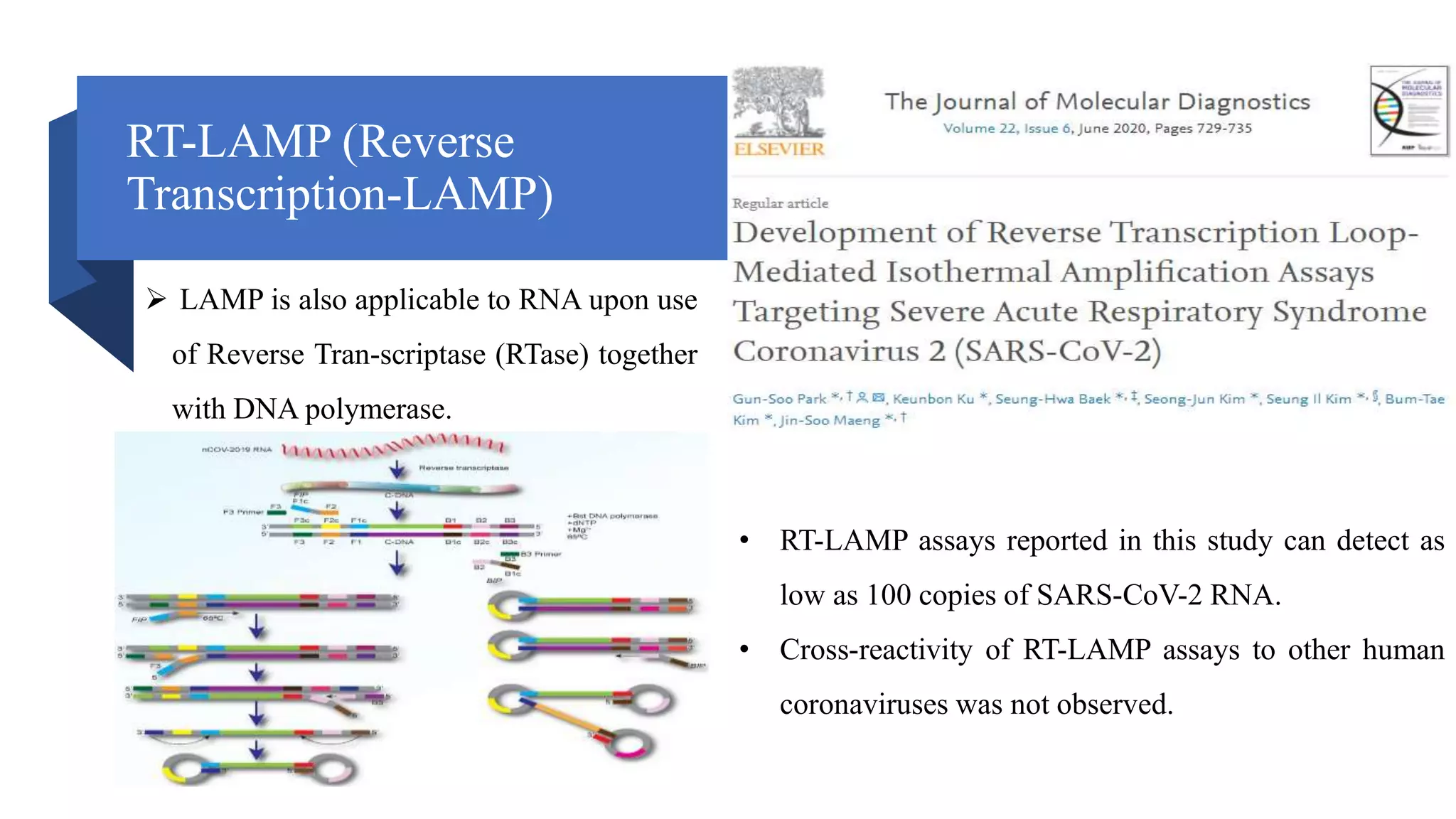
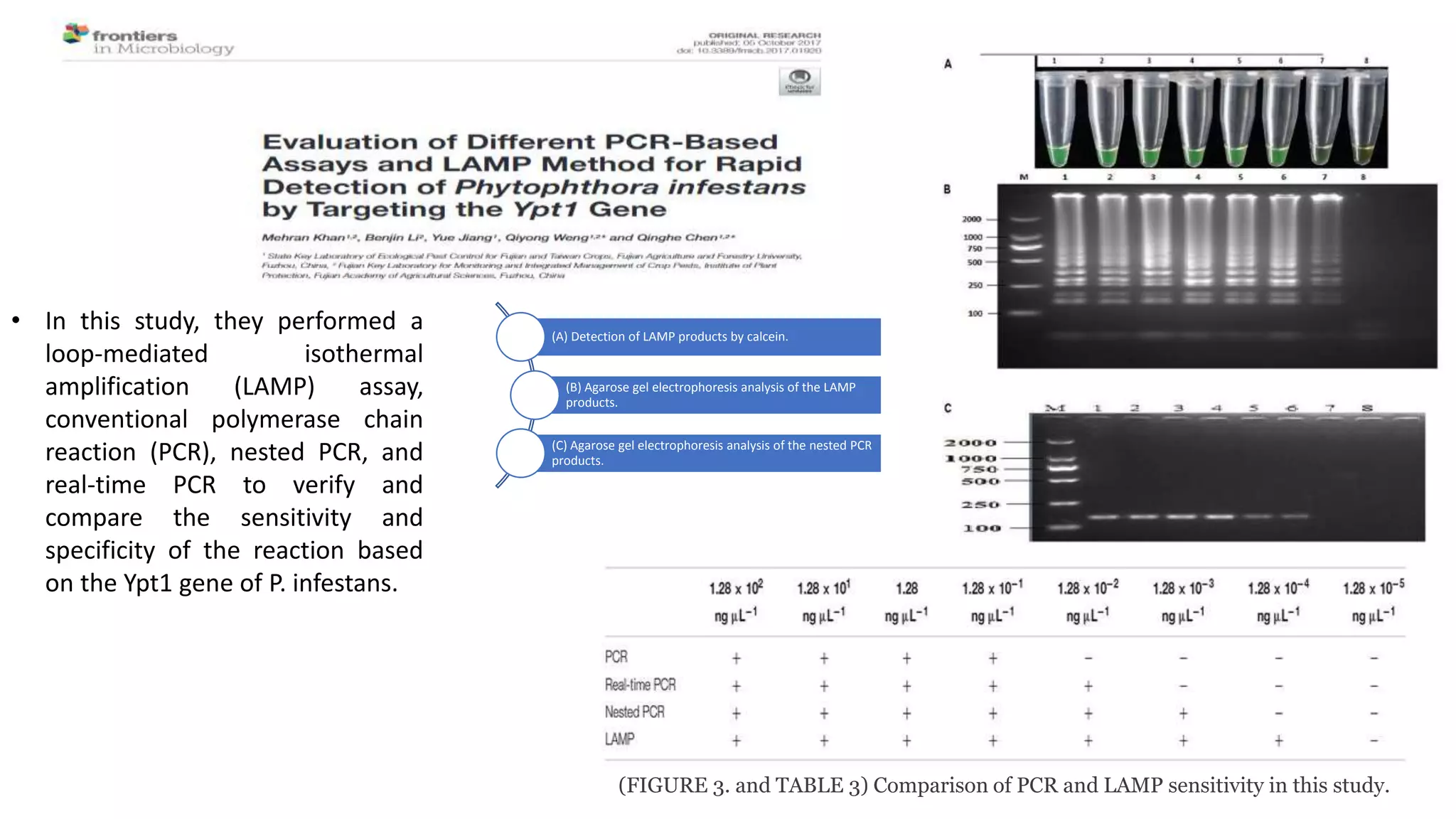
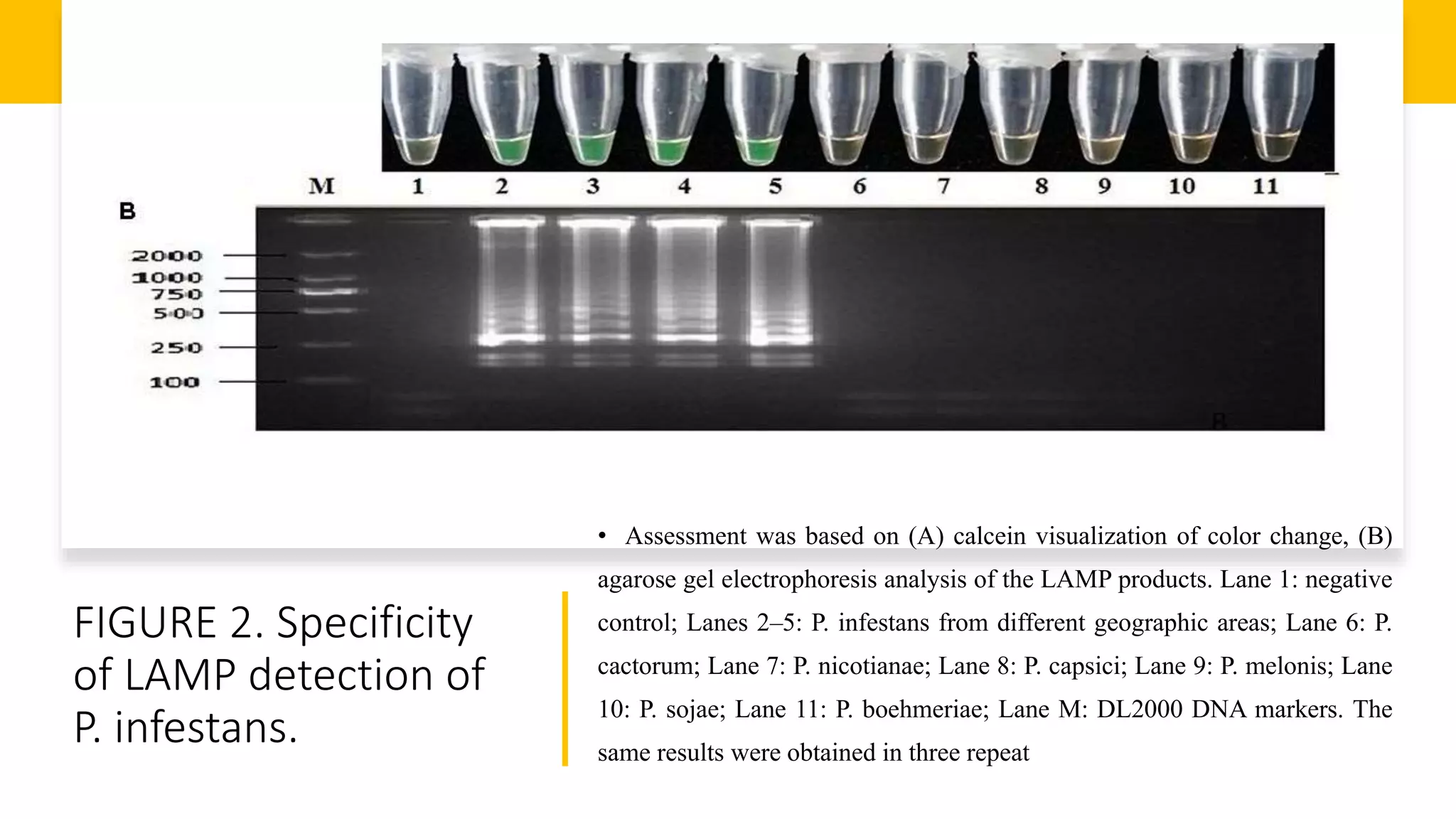
This document discusses loop-mediated isothermal amplification (LAMP), a DNA amplification technique. LAMP uses 4-6 specially designed primers to amplify DNA under isothermal conditions. It has advantages over PCR such as faster amplification time (30-60 minutes), constant reaction temperature, and simpler reaction setup. LAMP can detect as few as 6 copies of DNA and has been used to detect various pathogens. The document compares LAMP to PCR and other techniques and discusses LAMP primer design, reaction principles, visualization methods, advantages, and limitations.