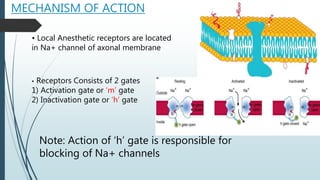
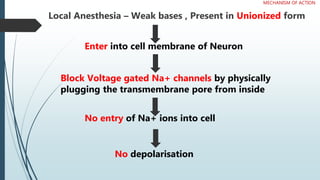
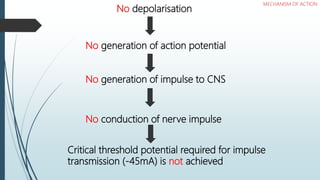
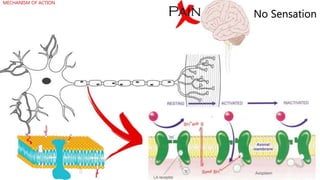
Local anesthetics work by reversibly blocking sodium channels in nerve cell membranes, preventing the transmission of nerve impulses and sensation in a localized area. The three main classes are ester anesthetics like procaine, amide anesthetics like lidocaine, and ether/ketone anesthetics like pramocaine. Amide anesthetics have a faster onset and longer duration than esters. Local anesthetics work topically on mucous membranes or can be injected for nerve blocks. The mechanism involves blocking sodium channels from the inside of nerve cells. Overdoses can cause CNS and cardiac side effects.





![Pharmacokinetics :
Not effective due to its poor penetration through GI
mucosa and high first pass effect
parenterally injection → has delayed onset of action ( 5-10
min) and short duration of action ( 25-30 min )
[ causes vasodilation → results in rapid absorption and
biotransformation ]
after absorption → rapidly hydrolysed by plasma
cholinesterase and by esterase in liver → inactive
metabolites PABA and diethylaminoethanol → excreted by
the kidneys into the urine.
Metabolism : Hydrolysis by plasma esterases to PABA
Route of elimination : With normal kidney function, the
drug is excreted rapidly by tubular excretion.
Half life : 7.7 minutes](https://image.slidesharecdn.com/484950localanesthesia-190712081509/85/Local-anesthesia-11-320.jpg)