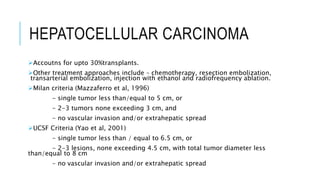
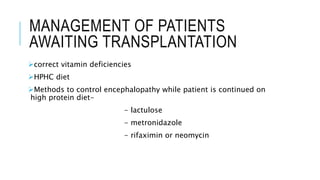
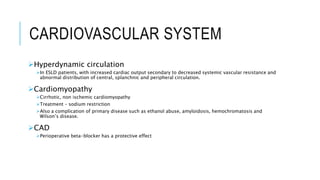
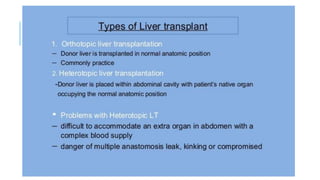
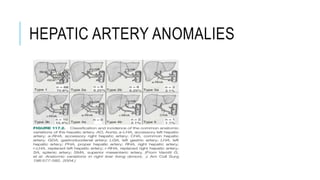
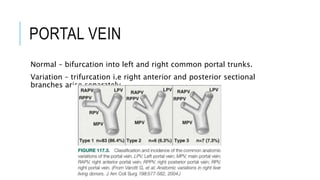
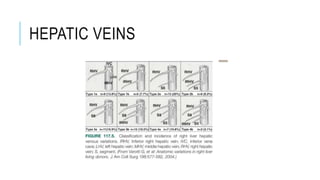
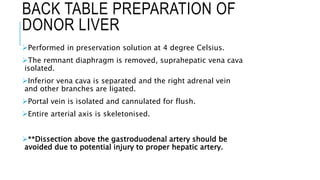
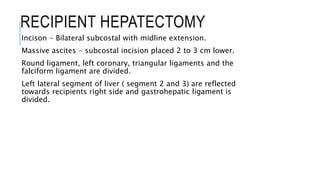
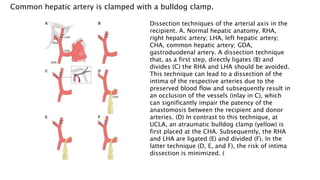
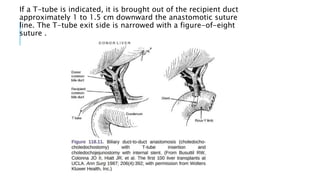
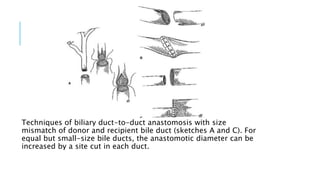
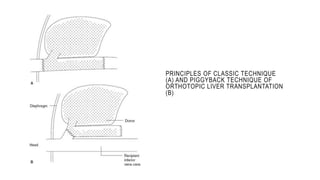
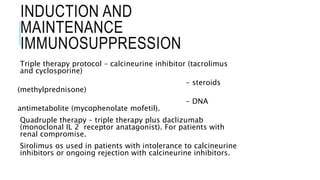
The document provides an extensive overview of liver transplantation, detailing its history, types of donors and grafts, organ viability, and ethical issues. It discusses various clinical considerations, indications, contraindications, and outcomes in liver transplantation, along with specific attention to pediatric transplantation and the management of patients awaiting surgery. Additionally, it examines the regulatory aspects of organ transplant laws, particularly in India, and outlines the assessment processes for candidates and donors in the transplantation framework.