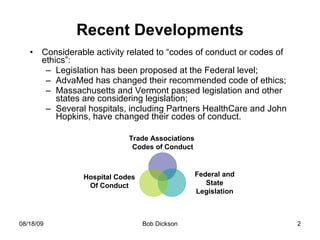
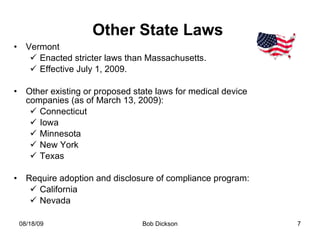
The document summarizes recent developments regarding codes of conduct for interactions between medical device companies and healthcare providers, including new legislation in Massachusetts. Key points include:
- Massachusetts passed a law requiring medical device companies to adopt a marketing code of conduct and disclose payments to healthcare providers.
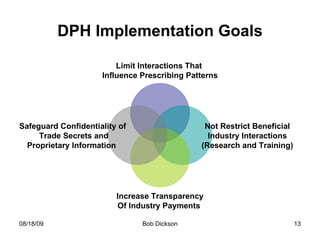
- The Massachusetts Department of Health issued regulations establishing prohibitions on gifts, meals and payments, with some exceptions for clinical trials, research and training.
- Medical device companies must implement compliance programs, conduct annual audits, and certify compliance with Massachusetts' marketing code to the Department of Health.