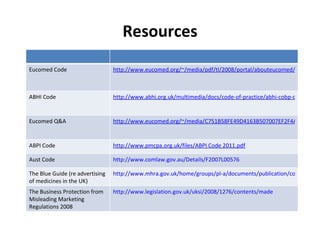
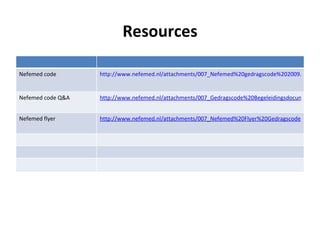
The document provides an overview of regulations and guidelines around the promotion of medical devices in Germany, the Netherlands and the UK. It discusses regulatory requirements, misleading and comparative advertising laws, industry codes of practice, and alternatives to legal action such as making complaints to regulatory or advertising standards authorities. Key points covered include the need for CE marking, restrictions on claims, guidelines around providing information versus promotional materials, and dealing with competitors' advertising claims.
![Life Sciences Seminar Germany, the Netherlands and the UK Alex Denoon, Mathias Klümper & Erik Vollebregt ©2009, Greenberg Traurig, LLP. Attorneys at Law. All rights reserved. GREENBERG TRAURIG, LLP ▪ ATTORNEYS AT LAW ▪ WWW.GTLAW.COM [ June 8, 2011 ] A Guide to Promotion of Medical Devices](https://image.slidesharecdn.com/presentationrepromotion8june20112-110613125810-phpapp02/75/Presentation-re-promotion-8-june-2011-2-1-2048.jpg)

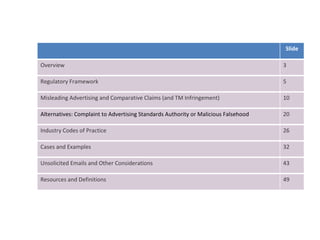
![Medical Device Promotion Regulatory Only approved devices within approved indications Unfair Commercial Practices Directive Local Implementation Inconsistent IP issues – especially Trade Mark Infringement and Unfair comparisons Unfair advantage L ’Oréal SA v. Bellure NV [2010] EWCA Civ 535 Industry Codes of Practice (EUCOMED, ABHI, NEFEMED and ABPI) Public Procurement considerations (if any) Professional body restrictions (e.g. HCP testimonials) Unsolicited emails](https://image.slidesharecdn.com/presentationrepromotion8june20112-110613125810-phpapp02/85/Presentation-re-promotion-8-june-2011-2-4-320.jpg)










































![Thank You The information in this presentation is provided for information purposes only. The information is not exhaustive. While every endeavour is made to ensure that the information is correct at the time of publication, the legal position may change as a result of matters including new legislative developments, new case law, local implementation variations or other developments. The information does not take into account the specifics of any person's position and may be wholly inappropriate for your particular circumstances. The information is not intended to be legal advice, cannot be relied on as legal advice and should not be a substitute for legal advice. Unless otherwise stated, the information is limited to the position in the Germany, the Netherlands and the UK. Alex Denoon: Mathias Klümper: +44 20 3441 0908 (office) +49 40 987613 28 (office) +44 7540 123 519 (mobile) +49 151 15138615 (mobile) [email_address] [email_address] Erik Vollebregt: +31 20 30 17 436 (office) +31 6 47 180 683 (mobile) [email_address]](https://image.slidesharecdn.com/presentationrepromotion8june20112-110613125810-phpapp02/85/Presentation-re-promotion-8-june-2011-2-49-320.jpg)