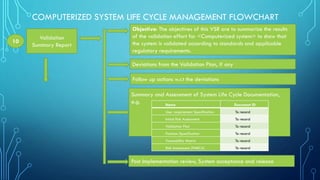
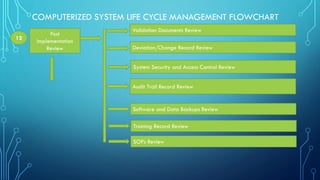
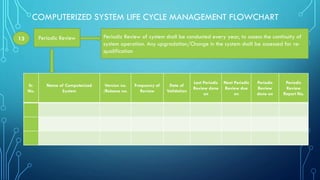
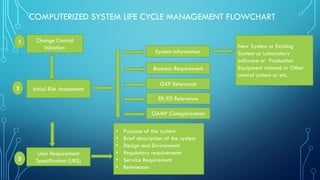
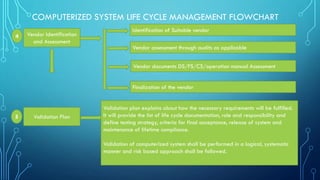
The document outlines the major steps in the life cycle management of computerized systems: requirements, development, validation, implementation/deployment, control, maintenance, and retirement. It then details the life cycle management flowchart which includes initiation and risk assessment, requirements specification, vendor identification and assessment, validation planning, system installation qualification, operation qualification, performance qualification, validation summary report, traceability matrix, post-implementation review, periodic review, revalidation for major changes, and retirement of outdated systems. The flowchart provides a systematic approach to qualifying computerized systems from initial requirements through maintenance and retirement.


![COMPUTERIZED SYSTEM LIFE CYCLE MANAGEMENT FLOWCHART
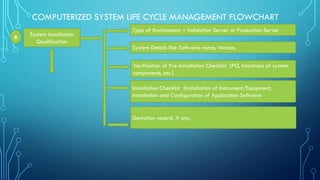
System Operation
Qualification
Roles of different personnel involved in the qualification of system
Reference documents to perform OQ
Test Planning [Test Items, Components and functions to be tested]
Test Strategy [Test Objective, Test Approach, Recording of results,
Acceptance criteria]
Deviation record, if any
Summary and conclusion
8](https://image.slidesharecdn.com/lifecyclemanagementofcomputerizedsystem-221122162502-36b4ba85/85/Life-Cycle-Management-of-Computerized-System-pdf-7-320.jpg)
![COMPUTERIZED SYSTEM LIFE CYCLE MANAGEMENT FLOWCHART
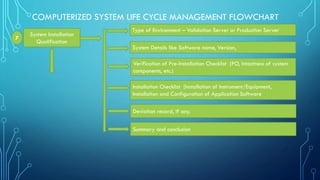
System Performance
Qualification
Roles of different personnel involved in the qualification of system
Reference documents to perform PQ
Test Planning [Test Items, Components and functions to be tested]
Test Strategy [Test Objective, Test Approach, Recording of results,
Acceptance criteria]
Deviation record, if any
Summary and conclusion
9](https://image.slidesharecdn.com/lifecyclemanagementofcomputerizedsystem-221122162502-36b4ba85/85/Life-Cycle-Management-of-Computerized-System-pdf-8-320.jpg)