Lesson 6 Unit C - Carboxylic Acids and Esters (1).pptx
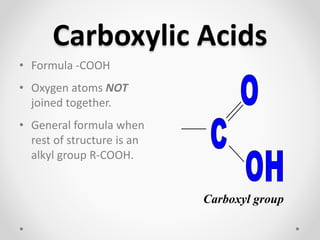
- 1. Carboxylic Acids • Formula -COOH • Oxygen atoms NOT joined together. • General formula when rest of structure is an alkyl group R-COOH. Carboxyl group
- 2. STRUCTURE OF CARBOXYLIC ACIDS • contain the carboxyl functional group -COOH • the bonds are in a planar arrangement • include a carbonyl (C=O) group and a hydroxyl (O-H) group
- 3. METHANOIC ACID ETHANOIC ACID PROPANOIC ACID Carboxylic acids form a homologous series BUTANOIC ACID 2-METHYLPROPANOIC ACID
- 4. Acids are named according to standard IUPAC rules 1. select the longest chain of C atoms containing the -COOH group 2. remove the “-e” and add “-oic” acid after the basic name 3. number the chain starting from the end nearer the COOH group 4. as in alkanes, prefix with alkyl substituents 5. side chain positions are based on the C in -COOH being 1 e.g. CH3 - CH(CH3) - CH2 - CH2 - COOH 4-methylpentanoic acid NAMING CARBOXYLIC ACIDS
- 5. Naming Practice • Name these structures Butanoic acid Octanoic acid Pentanedioic acid
- 6. NAMING CARBOXYLIC ACIDS Many carboxylic acids are still known under their trivial names, some having been called after characteristic properties or their origin. Formula Systematic name (trivial name) origin of name HCOOH methanoic acid formic acid latin for ant CH3COOH ethanoic acid acetic acid latin for vinegar C6H5COOH benzenecarboxylic acid benzoic acid from benzene 101°C 118°C 141°C 164°C PHYSICAL PROPERTIES BOILING POINT Increases as size increases - due to increased van der Waals forces
- 7. PHYSICAL PROPERTIES BOILING POINT Carboxylic acids have high boiling points for their relative mass arises from inter-molecular hydrogen bonding due to polar O—H bonds The effect of hydrogen bonding on the boiling point of compounds of similar mass Compound Formula Mr b. pt. (°C) Comments ethanoic acid CH3COOH 60 118 propan-1-ol C3H7OH 60 97 H-bonding propanal C2H5CHO 58 49 dipole-dipole butane C4H10 58 - 0.5 basic V der W
- 8. PHYSICAL PROPERTIES cont… SOLUBILITY • carboxylic acids are soluble in organic solvents • they are also soluble in water due to hydrogen bonding HYDROGEN BONDING • small ones dissolve readily in cold water • as mass increases, the solubility decreases
- 9. Carboxylic Acids • Carboxylic acids exhibit dipole-dipole interactions because they have polar C=O and O—H bonds. • They also exhibit intermolecular hydrogen bonding. • Carboxylic acids are held together by two intermolecular hydrogen bonds. Physical Properties
- 10. Carboxylic Acids Interesting Carboxylic Acids Butanoic acid is an oxidation product that contributes to the disagreeable smell of body odor. It’s common name, butyric acid, is derived from the Latin word butyrum, meaning “butter”, because butyric acid gives rancid butter its peculiar odor and taste. Acetic acid is the sour component of vinegar. The air oxidation of ethanol to acetic acid is the process that makes “bad” wine taste sour (acidus). Acetic acid is an industrial starting material for polymers used in paints and adhesives. Formic acid has an acrid odor and a biting taste, and is responsible for the sting of some types of ants. The name is derived from the Latin word formica, meaning “ant”.
- 11. More Interesting Carboxylic Acids Caproic acid, the common name for hexanoic acid, has the foul odor associated with dirty socks and locker rooms. Its name is derived from the Latin word caper, meaning “goat”. Oxalic acid (Rhubarb) and lactic acid are simple carboxylic acids that are quite prevalent in nature. Salts of carboxylic acids are commonly used as preservatives. Sodium benzoate is a preservative used in soft drinks and baked goods.
- 12. These red ants, like other ants, make the simplest of the organic acids, formic acid. The sting of bees, ants, and some plants contains formic acid, along with some other irritating materials. Formic acid (methanoic acid) is HCOOH. Salad Dressing?
- 13. PREPARATION OF ESTERS(Esterification) Reagent(s) alcohol + carboxylic acid Conditions reflux with a strong acid catalyst (e.g. conc. H2SO4 ) Equation e.g.) CH3CH2OH(l) + CH3COOH(l) CH3COOC2H5(l) + H2O(l) ethanol ethanoic acid ethyl ethanoate Notes Conc. H2SO4 is a dehydrating agent - it removes water causing the equilibrium to move to the right and thus increases the yield of the ester CH3 C O CH2 CH3 O Acid part of ester Alcohol part of ester
- 14. ETHYL METHANOATE METHYL ETHANOATE Naming Esters: Named from the original alcohol and carboxylic acid CH3OH(l) + CH3COOH(l) CH3COOCH3 (l) + H2O(l) from ethanoic acid CH3COOCH3 from methanol METHYL ETHANOATE
- 15. CH3 C O CH2 CH3 O ethyl ethanoate (IUPAC) CH3 CH C O CH3 CH3 O methyl 2-methylpropanoate
- 16. ethyl pentanoate Br O Et O 1 2 3 4 5 6 CH3 CH2 CH2 CH2 C O CH2 CH3 O ethyl 5-bromo-3,4-dimethylhexanoate DON’T Have To Know!
- 17. Esters as Flavoring Substances Isopentyl acetate Ethyl butyrate banana pineapple Isobutyl propionate Octyl acetate rum orange Propyl acetate Methyl butyrate pear apple CH3 C O CH2 CH2 CH3 O CH3 CH2 C O CH2 CH CH3 O CH3 CH3 C O CH2 CH2 CH2 O CH2 CH2 CH2 CH2 CH3 CH3 CH2 CH2 C O CH2 CH3 O CH3 C O CH2 CH2 CH O CH3 CH3 CH3 CH2 CH2 C O CH3 O Solvents: nail varnish remover - ethyl ethanoate octyl ethanoate ethyl butanoate propyl ethanoate methyl butanoate NOT IUPAC!
- 18. SUMMARY OF THE CLASSES OF ORGANIC COMPOUNDS • alkanes – single bonds • alkenes – double bond • alkynes – triple bond • aromatic – benzene ring C6H5 – • organic halides – halogen R = hydrocarbon chain/group R – X (X = halogen) • alcohols – hydroxyl group R(H) = hydrocarbon chain/group or can be hydrogen R – OH • carboxylic acids – carboxyl group R – COOH • esters – ester group –COOC– R1(H)COOCR2