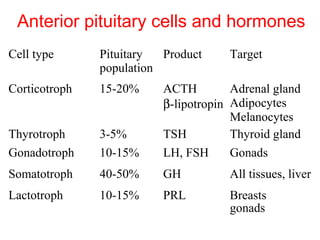
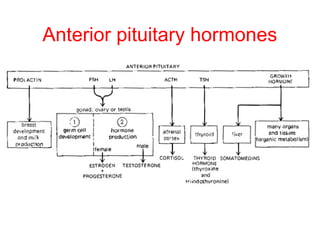
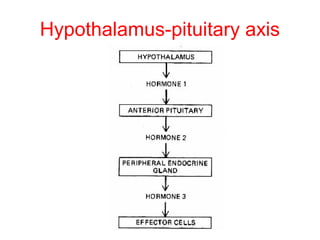
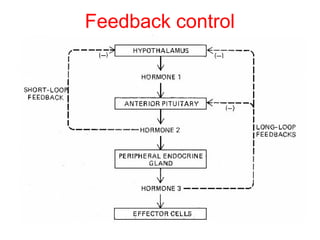
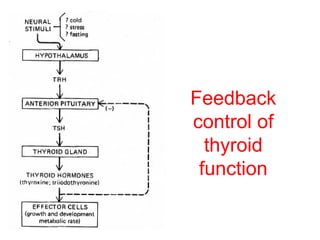
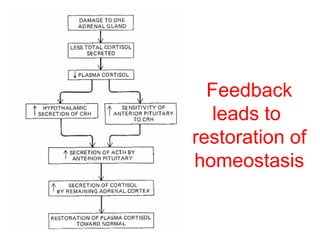
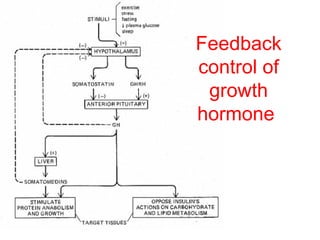
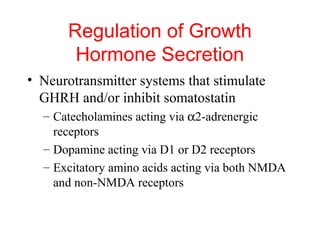
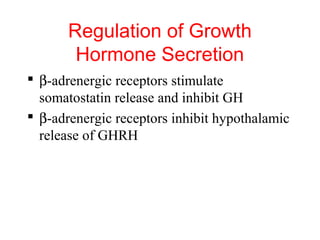
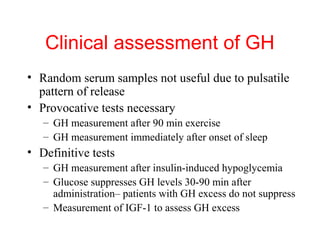
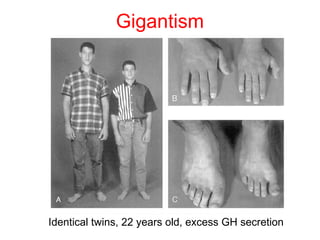
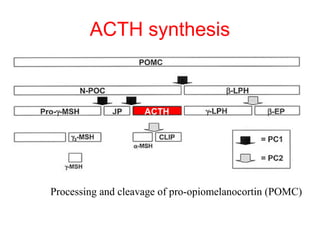
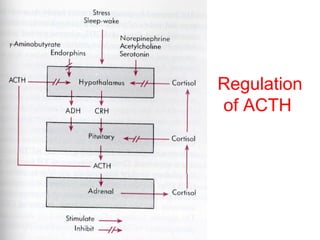
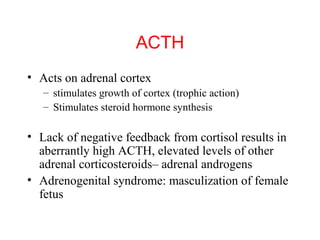
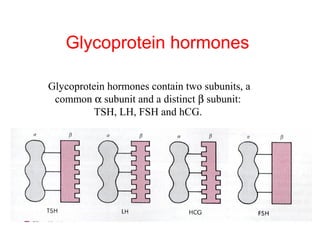
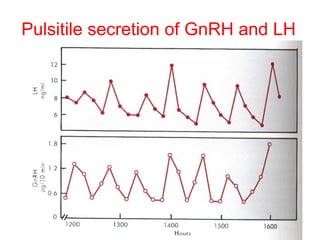
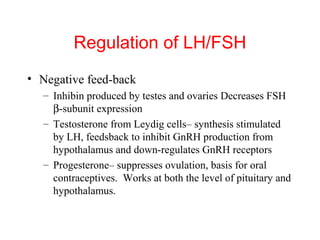
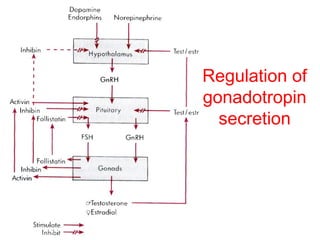
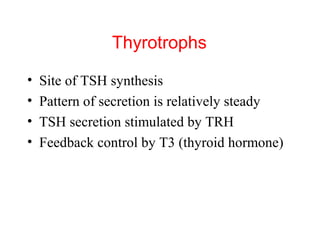
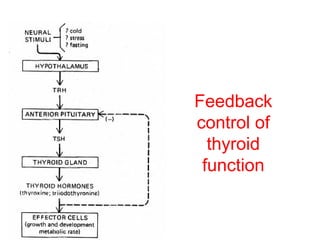
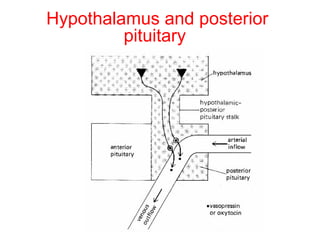
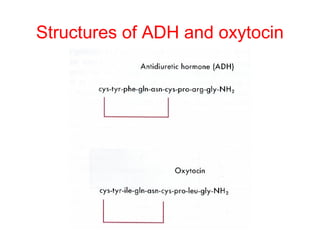
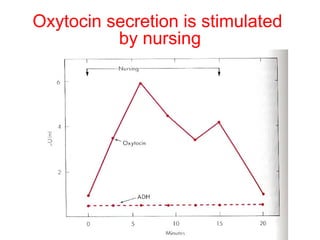
The anterior pituitary produces six hormones: prolactin, growth hormone, thyroid stimulating hormone, adrenocorticotropic hormone, follicle-stimulating hormone, and luteinizing hormone. These hormones are regulated by feedback from their target organs/tissues. Negative feedback maintains homeostasis by inhibiting hormone production when levels are sufficient. The hypothalamus controls the anterior pituitary through releasing and inhibiting hormones like CRH and somatostatin.