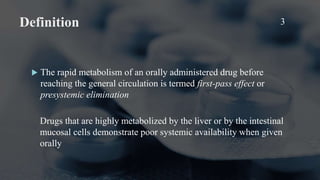
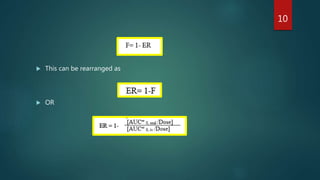
The document discusses the first-pass effect and hepatic elimination of orally administered drugs. It defines the first-pass effect as the rapid metabolism of a drug by the liver or intestinal mucosa before it reaches systemic circulation. This results in lower bioavailability compared to intravenous administration. The liver extraction ratio measures the liver's efficiency in eliminating a drug from the bloodstream during a single pass through the liver. It can be estimated from a drug's oral bioavailability and provides information about the liver's extraction of the drug and implications for oral dosing. Drugs with high liver extraction require higher oral doses to achieve equivalent effects as intravenous administration due to the first-pass effect.