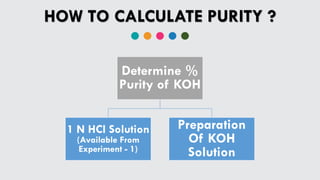
Experiment 1 standardized a 500 ml HCl solution against 1 N NaOH solution. The HCl solution was titrated with 1 N NaOH using a burette and phenolphthalein indicator. Calculations using the titration data found the HCl solution to be 1.015 N.
Experiment 2 determined the % purity of a KOH sample. A KOH solution was prepared from a 2 gm sample and titrated against the 1.015 N HCl solution from Experiment 1 using methyl orange indicator. Calculations based on the titration data found the % purity of the KOH sample to be 84.65%, close to the expected 84% purity.