This document describes an experiment to synthesize copolymers of myrcene with maleic anhydride or tert-butyl acrylate using nitroxide mediated polymerization with NHS-BlocBuilder initiator. Myrcene/maleic anhydride copolymerization was unsuccessful likely due to a side Diels-Alder reaction between the monomers. Myrcene/tert-butyl acrylate copolymerizations with varying monomer feed compositions were more controlled. Analysis of the copolymers found decreasing molecular weight and low polydispersity as the tert-butyl acrylate content increased, possibly due to intramolecular backbiting terminations.



![4
2. Introduction
With the fast development of the modern technologies, the demands for materials with high
performance and versatility have been dramatically increased in the last few decades. Polymers,
due to their mechanical flexibility and low production costs, have contributed a considerably
portion to the synthesis of these materials. While the current chemical processes of polymer
synthesis are mainly based on the bulk chemicals such as carbon monoxide, hydrogen, ethylene,
propylene and benzene, obtained from oil and gas[1]
, there is an increasing emphasis to use
monomers from sustainable resources like myrcene[2]
and pinene[3]
. In particular, poly(myrcene)
P(My), exhibiting a significant average molar mass, behaves like a rubber such as poly(isoprene)
or poly(butadiene). However, due to myrcene’s non-polar structure (Figure 1), the adhesion and
reactive blending for example of poly(myrcene) with polar polymeric materials are not
remarkable. To compensate this defect and expand its usage, certain polar monomers need to
be copolymerized with myrcene. Functional groups like anhydrides (borne by MA) or carboxylic
acids (borne by acrylic acid) can be copolymerized with non-polar monomers such as myrcene or
isoprene. In the first part of the project, maleic anhydride bearing anhydride functional group
was going to copolymerize with myrcene. In the second part, tert-butyl acrylate (Figure 1) was
going to be introduced to copolymerize with myrcene. Note that we used tert-butyl acrylate
(tBuA) instead of acrylic acid (AA). Indeed, the sensitivity of the SG1 free nitroxide toward
strong acids such as AA is problematic (SG1 consumed in degradative side reactions with AA).
Rather than copolymerizing AA directly with myrcene, it is more facile to first copolymerize
tBuA with My, followed by cleavage of the protecting group to yield AA functional groups (well-
known and efficient deprotection with trifluoroacetic acid).
In order to achieve the two different copolymers exhibiting desirable average molecular
weights and a high degree of livingness (capability of the copolymer to re-initiate a fresh batch
of monomer), the copolymerizations must proceed in a controlled manner. One traditional
method was ionic polymerization, in which a strong base (anionic polymerization) or a strong
acid (cationic polymerization) served as the initiator, and thanks to the strong reactivity of the
ions, each polymer chain can be initiated and propagate simultaneously, which achieves the
mono-dispersity of the polymers. However these highly reactive ions also increased the
sensitivity to solvent, temperature and impurities[4]
, leading to an increase in the cost for
purification and in the difficulties for the experimental operation. In addition, ionic
Figure 1: Myrcene (left), maleic anhydride (middle) and tert-butyl acrylate (right) monomers.](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-4-2048.jpg)
![5
polymerization is restricted to monomers which do not have interference on the polymerization.
For example, monomers with strong acidic groups tend to react with the counter-ions which
could cause the failure in terminating the reaction. The acrylic acid group in tert-butyl-acrylate is
one of these group. Therefore, the ionic polymerization method could not be applied in this
experiment[5]
. One alternative method that could also proceed the copolymerization in a
controlled manner is termed controlled radical polymerization. The controlled radical
polymerization combines the control of microstructure from ionic polymerization and the ease
of industrial implementation from other types of polymerizations such as free radical
polymerization or addition polymerization[5]
. Thus using controlled radical polymerization could
avoid the rigorous requirements on the solvent, temperature and impurities that ionic
polymerization had, and it has proven to be capable of polymerizingmyrcene and tert-butyl-
acrylate[5]
.
There are three major types of living/controlled polymerization termed nitroxide-mediated
radical polymerization (NMP), atom-transfer radical polymerization (ATRP), and reversible
addition-fragmentation chain-transfer polymerization (RAFT). Because of the poisoning of ATRP
catalyst by acrylic acid, ATRP cannot be applied here, and RAFT requires the chain transfer
agents that are not currently commercially available. Therefore, among the three variants, NMP
was going to be applied in this experiment[5]
.
Depending on the type of monomers being polymerized, the initiators used in NMP can be
different. For example, first-generation nitroxide mediators such as TEMPO (2,2,6,6-
tetramethylpiperidinyl-1-oxy) are used for the polymerization of mostly styrenic-based
monomers, and it is not effective to control the polymerization of acrylates and methacrylate[6].
In this experiment, NHS-BB (Figure 2) is going to be used as it has been proven to be capable of
controlling the homopolymerization of butyl acrylates.
Figure 2: NHS-BlocBuilder (left) and SG1 free nitroxide (right)[6]
.
In order to sufficiently understand the kinetics and analyze the results, the overall conversions
of the monomers and the individual conversions of myrcene and tert-butyl-acrylate (or maleic
anhydride) were determined. In this project, the overall conversions were achieved by
measuring the empty vial weight, the vial weight with monomer solution and polymer inside
(wet vial weight) and the vial weight after completely drying out the monomer solution (dry vial
weight) and calculating the gravimetric differences. The gravimetric difference of dry vial weight
and empty vial weight gives the mass of polymer (product), and the gravimetric difference of
wet vial weight and empty vial weight gives the mass of monomers and polymer (product +](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-5-2048.jpg)

![7
3. Experimental Procedure
3.1. Materials
Basic alumina (Brockmann, Type 1, 150 mesh) and calcium hydride (90-95% reagent grade)
were purchased from Aldrich. 2-Methyl-2-[N-tert-butyl-N-(1-diethoxyphosphoryl-2,2-
dimethylpropyl)-aminoxy]–N-propionyloxy-succinimide, also known as NHS-BlocBuilder, was
synthesized in the laboratory from 2-{tert-Butyl[1-(diethoxyphosphoryl)-2,2-
dimethylpropyl]aminogoxy)-2-methylpropanoic acid, also known as BlocBuilderTM
(MAMA-SG1,
99%, acquired from Arkemaand used without further purification), N-hydroxy-succinimide(NHS,
98%, from Aldrich and used as received) and N,N’-dicyclohexylcarbodiimide(DCC, 99%, from
Aldrich and used as received). Myrcene(My, ⩾90%) and maleic anhydride (MA, 99%) were
obtained from Sigma-Aldrich and used as received. Tert-butyl acrylate (tBuA, 98%) obtained
from Aldrich was purified by passage through a column of calcium hydride and basic alumina
mixture (1:20 by weight, respectively) and stored in a sealed flask in a refrigerator under a head
of nitrogen. Methanol (MeOH, 99.9%) was obtained from Fisher Scientificand used as received.1,
4-dioxane (ACS reagent, >99.0%) was obtained from Sigma-Aldrich and used as
received.Chloroform-d1(CDCl3, 99.8%) for NMR measurements was purchased from Merck
KGaA. Tetrahydrofuran (THF, 99.9%, HPLC grade) for GPC measurements was purchased from
Fischer Scientific.
3.2. Syntheses
3.2.1. Initial feed for myrcene/maleic anhydride
Copolymerization
Two polymerizations were performed with different initial myrcene and maleic anhydride molar
compositions. The feed conditions were listed in Table 1. Because maleic anhydride is solid at
room temperature unlike myrcene at the liquid state, 1,4-dioxane was added as solvent to
dissolve maleic anhydride monomer and ensure a homogeneous reaction medium (myrcene
totally miscible in this solvent). The target number average molecular weight (Mn,target) at 100%
overall conversion was approximately 30 kg/mol and was calculated by the initial moles of
monomers relative to the moles of NHS-BlocBuilder initiator.
Table 1 Experimental conditions myrcene /maleic anhydride copolymerization
in 1,4-dioxane at 115℃ using NHS-Blocbuilder as initiator.
Experiment
ID
[NHS-
Blocbuilder]0
[Myrcene]0
[Maleic
Anhydride]0
[1,4-
dioxane]
fmyrcene
a Mn-
,target
b
mol L-1
mol L-1
mol L-1
mol L-1
mol%
kg
mol-1
My-MA
90-10
0.017 2.133 2.133 5.761 50 30
My-MA
50-50
0.015 2.981 0.331 5.454 90 30](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-7-2048.jpg)
![8
a
Initial molar composition with respect of myrcene. b
Number average molecular weight at
100% overall conversion determined by gel permeation chromatography (GPC).
3.2.2. Initial feed for myrcene/tert-butyl acrylate
copolymerization
Four copolymerizations with the use of different myrcene and tert-butyl acrylate monomer
compositions were performed as shown in Table 2. The monomers were purified by basic
alumina and calcium hydride. NHS-BlocBuilder was used as initiator and the amount was set
constantly to 0.25g for each case. The target number average molecular weight of the final
product at 100% overall conversion was approximately 40 kg/mol and was calculated by the
moles of monomers relative to the moles of NHS-BlocBuilder initiator.
Table 2 Experimental conditions of the bulk copolymerizations of myrcene
and tert-butyl acrylate at 115℃ using NHS-Blocbuilder as initiator
Experiment
ID
[NHS-Blocbuilder]0 [Myrcene]0 [Tert-Butyl acrylate]0 fmyrcene
a
Mn,target
b
mol L-1
mol L-1
mol L-1
mol% kg mol-1
My-tBuA
0-100
0.022 0.000 6.889 0 40
My-tBuA
50-50
0.021 3.157 3.157 50 40
My-tBuA
70-30
0.020 4.277 1.833 70 40
My-tBuA
90-10
0.020 5.328 0.592 90 40
a
Initial feed molar composition with respect of myrcene. b
Number average molecular weight
at 100% overall conversion.
3.2.3. General Synthesis Procedure
The syntheses were all performed in a 50 mL 3 neck round bottom glass flask equipped with a
condenser, thermal well and magnetic Teflon stir bar. The chilling liquid inside the condenser
was 90 v% water and 10 v% glycol, and the chilling temperature was set to 3 ℃ to sufficiently
condense any vapors produced in order to prevent from any monomer loss during the
experiment. The condenser was connected to the middle neck of the flask and was capped with
a rubber septum at the top which had a needle placed to relieve the pressure of the nitrogen
purge that was applied during the entire course of the reaction. The glass flask was placed inside
the heating mantle, which was equipped on the top of the magnetic stirrer. A specific
formulation for an initial feed composition of myrcene (fmyrcene,0) equal to 50 mol% was given as
an example. The masses of NHS-BlocBuilder (0.25 g, 0.523 mmol), myrcene (10.78 g, 79.124
mmol) and t-BuA (10.14 g, 79.124 mmol) were measured inside a disposable vial and transferred
into the reactor which was then sealed with a rubber septum (in the case of myrcene/maleic
anhydride copolymerization, 1,4-dioxane and myrcene were firstly measured and transferred
into the reactor, and the maleic anhydride was slowly added into the mixture due to the](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-8-2048.jpg)

![10
4. Results and discussion
4.1. Myrcene/maleic anhydride copolymerization
In general, copolymerization kinetics is characterized as 1st
-order kinetics as illustrated in
Equation 2, and the logarithm of 1/(1-x) should linearly increase as the reaction time increases.
Where M0 is the initial monomer concentration; Mt is the monomer concentration after time t;
<kp> is the average propagation rate constant; x is the overall conversion; [P∙] is the propagating
radical concentration and t is the time.
However, as shown in Figure 3, the experimental data with both an initial feed concentration of
50mol% myrcene (fmyrcene0 =0.5) and 90mol% myrcene(fmyrcene0 =0.9) do not steady increase in
ln(1/(1-x)) and instead, apparent fluctuations are presented in the series of data points. Besides,
surprisingly, at time of 0 min, very high conversions (around 30% for fmyrcene=0.9 and around 70%
for fmyrcene=0.5) are observed. Due to the strong contradictions between the experimental
observations and the general polymerization theories, there is a large possibility of the failure
on the copolymerization process of myrcene and maleic anhydride.
Figure 3: Semi-logarithmic kinetic plot of ln((1-x)-1) (x = overall conversion) versus time for various initial
myrcene/maleic anhydride (My/MA) molar compositions polymerized at 115 o
C.
One rationale of the unexpected results could be the occurrence of a side reaction, and the
most possible side reaction, considering the conditions of the experiments, is the Diels-Alder
reaction. Generally speaking, the Diels-Alder reaction is an organic chemical reaction between a
conjugated diene and a substituted alkene[7]
and the general form is illustrated in Figure 4.
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0 50 100 150 200 250 300
ln(1/(1-x))
Time (min)
My-MA 90-10
My-MA 50-50
My-MA 90-10
My-MA 90-10
ln (
𝑀0
𝑀𝑡
) = ln (
𝑀0
𝑀0(1 − 𝑥)
) = ln (
1
1 − 𝑥
) =< 𝑘 𝑝 > [𝑃 ∙]𝑡 Equation 2](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-10-2048.jpg)
![11
Figure 4: The Diels-Alder reaction.
In this experiment, myrcene (7-Methyl-3-methylene-1,6-octadiene) is a diene and maleic
anhydride (Furan-2,5-dione) bears a reactive double bond, which satisfy the conditions of the
Diels-Alder reaction. The plausible reaction equation is presented in Figure 5.
According to the literature, Diels-Alder reaction can happen at low temperature (40o
C) and
generate high yield of adduct (25%)[8]
. Therefore, during the heating up process, the Diels-Alder
reaction may have already started and converted significant amount of monomers before the
initiation of the copolymerization. Therefore, at time of 0min, which is in our case defined
arbitrarily when the temperature reaches 90o
C, a high conversion of monomers has been
achieved. In addition, kinetics of the Diels-Alder reaction is proven to be an overall 2nd
order as
shown in Equation 3[9]
, where [My] is the myrcene concentration and [MA] is the maleic
anhydride concentration.
When the total concentration of myrcene and maleic anhydride stays the same ([My] + [MA] = a
constant), the closer [My] and [MA] are to each other, the larger the product of them is, and the
higher the rate of production will be. This explains why the starting conversion is higher when
fmyrcene0 is 50% than when fmyrcene0 is 10%.
The other possible contribution to the errors may be due to the impurities, mainly ring
structure dienes, in myrcene monomer. In the literature[10]
, regarding isoprene/mA
copolymerization, the cyclopentadiene effectively inhibits the stereospecific polymerization of
isoprene if presents in amount of higher than 10ppm. Based on the similarities in the structure
between isoprene and myrcene, it could be hypothesized that similar inhibitions are possible to
happen during My/MA copolymerization, which involved the myrcene with only 90% purity.
𝑑𝑃
𝑑𝑡
= 𝑘[𝑀𝑦][𝑀𝐴] Equation 3
+
Figure 5: Schematic for mycene/maleic anhydride Diels-Alder reaction](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-11-2048.jpg)
![12
To avoid these failures due to the possible reasons mentioned above, several modifications on
the experimental procedure can be made. Firstly, using 4-methoxy-2,4-dimethylvaleronitrile
(AMVN) as the radical initiator and twisting the diene functional group to be “cis” instead of
“trans” has been proven to give high yield of copolymerization and effectively retard the Diels-
Alder side reaction[8]
. Secondly, the side product could be removed. For instance,
cyclopentadiene, side product achieved after copolymerizing isoprene with maleic anhydride,
can be removed. This resulting adduct is separated by decantation and distillation[10]
. Similar
principle could be applied to myrcene to remove the impurities the same way.
For fMA0 = 0.10, the copolymerization was very slow and uncontrolled. After 6h, the copolymer
exhibited Mn = 5 500g.mol-1 at 46% overall conversion instead of 14 000g.mol-1 theoretically. For
fMA0 = 0.50, it was even worse since no polymerization occurred (absence of polymer peak in the
GPC chromatograms).
4.2. Myrcene/tert-Butyl acrylate copolymerization
The kinetic results of My/tBuA copolymerization using NHS-Blocbuilder as the initiator are
presented in Figure 6. All copolymerization follow 1st
-order kinetic behavior and the formula can
be represented as Equation 2. Therefore, by plotting ln (
1
1−𝑥
) versus time, the slope would give
<kp> [P∙]. Since in controlled radical polymerization, [P∙] could be assumed to be equal to the
concentration of the initiator, so <kp><K> can be further obtained from dividing the slope by [P∙].
Figure 6: Semi-logarithmic kinetic plot of ln((1-x)-1
) (where x = overall conversion) versus time for various
myrcene/tert-butyl-acrylate (My/tBuA) compositions polymerized at 115 o
C.
0.0
0.2
0.4
0.6
0.8
1.0
1.2
0 100 200 300
ln(1/(1-x))
Time (min)
My-tBuA 0-100
My-tBuA 50-50
My-tBuA 70-30
My-tBuA 90-10
My-tBuA 0-100
My-tBuA 50-50
My-tBuA 70-30
My-tBuA 90-10](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-12-2048.jpg)
![13
Table 3 shows the values of different <kp><K> with different initial tBuA feed composition. It
should be noticed that as the initial feed composition of tBuA increased, the values of the slopes
raised up, which indicates an increase in <kp><K>. Therefore, the propagation rate of myrcene is
significantly lower than the propagation rate of tBuA. A most dramatic increase is from ftBuA0 =
90% to ftBuA0 = 100% (from 0.1330 to 0.2556), and the <kp><K> does not change much from ftBuA0
= 90% to ftBuA0 = 100% (from 0.1140 to 0.1330).
Table 3 Slopes of Semi-Logarithm Plot of ln ((1-x)-1
) Versus Time for Various
Initial tBuA Feed Compositions
Experiment ID
ftBuA
a
Slopes <kp><K>
mol% L mol-1
s-1
s-1
My-tBuA 50-50 50 0.01464 0.06654
My-tBuA70-30 70 0.002395 0.1140
My-tBuA 90-10 90 0.002659 0.1330
My-tBuA 0-100 100 0.005113 0.2556
a
Initial feed composition with respect oftert-butyl-acrylate.
In Table 4, it is shown that from 0 minute to 15 minutes, there is a dramatic increase in the
conversion of tBuA (from 8.2% to 35.0%) while the increase in myrcene conversion is not as
significant (from 2.4% to 6.8%). Even though the initial feed composition of tBuA (10%) is much
lower than that of myrcene (90%), the propagation of tBuA monomers is still favored. This fact
also evidentially shows a favor on propagating tBuA, more reactive, rather than myrcene.
Table 4: Individual Myrcene and tBuA conversions at Different Times for 10%
tBuA Initial Feed Composition
Time [min] Myrcene Conversion [%] tBuA Conversion [%]
0 2.4 8.2
15 6.8 35.0
30 15.1 46.9
60 24.6 69.8
90 33.4 82.4
150 38.8 93.1
240 49.7 98.7
360 57.5 100.0
Linear average molecular weight increases with respect to the increase in overall conversion and
narrow molecular weight distribution are the characteristics generally associated with
controlled radical polymerizations. Figure 7 shows the plot of number average molecular weight
versus conversion with initial feed composition with respect to tBuA (ftBuA) of 100%. The
experimental Mn versus conversion data values are directly obtained from the GPC machine and](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-13-2048.jpg)
![14
the corrected Mn versus conversion values are obtained by utilizing the Mark-Houwink equation
to rectify the experimental data. In this feed condition, it is observed that the experimental
results tightly follow the theoretical predicted line below the conversion of 40% and the slope
start to drop afterwards. However, this deviation in slopes is not very significant and can be
attributed to the differences in hydrodynamic volumes of poly-tert-butylacrylate and that of the
polystyrene standards that used to calibrate the GPC[6]
. After the correction, the slope was
adjusted and raised up, which decreased the deviation, which indicates a well-controlled
copolymerization condition for tBuA polymerization.
Figure 7: Number average molecular weight (Mn) versus conversion with initial feed composition with
respect to tBuA (ftBuA) of 100%.
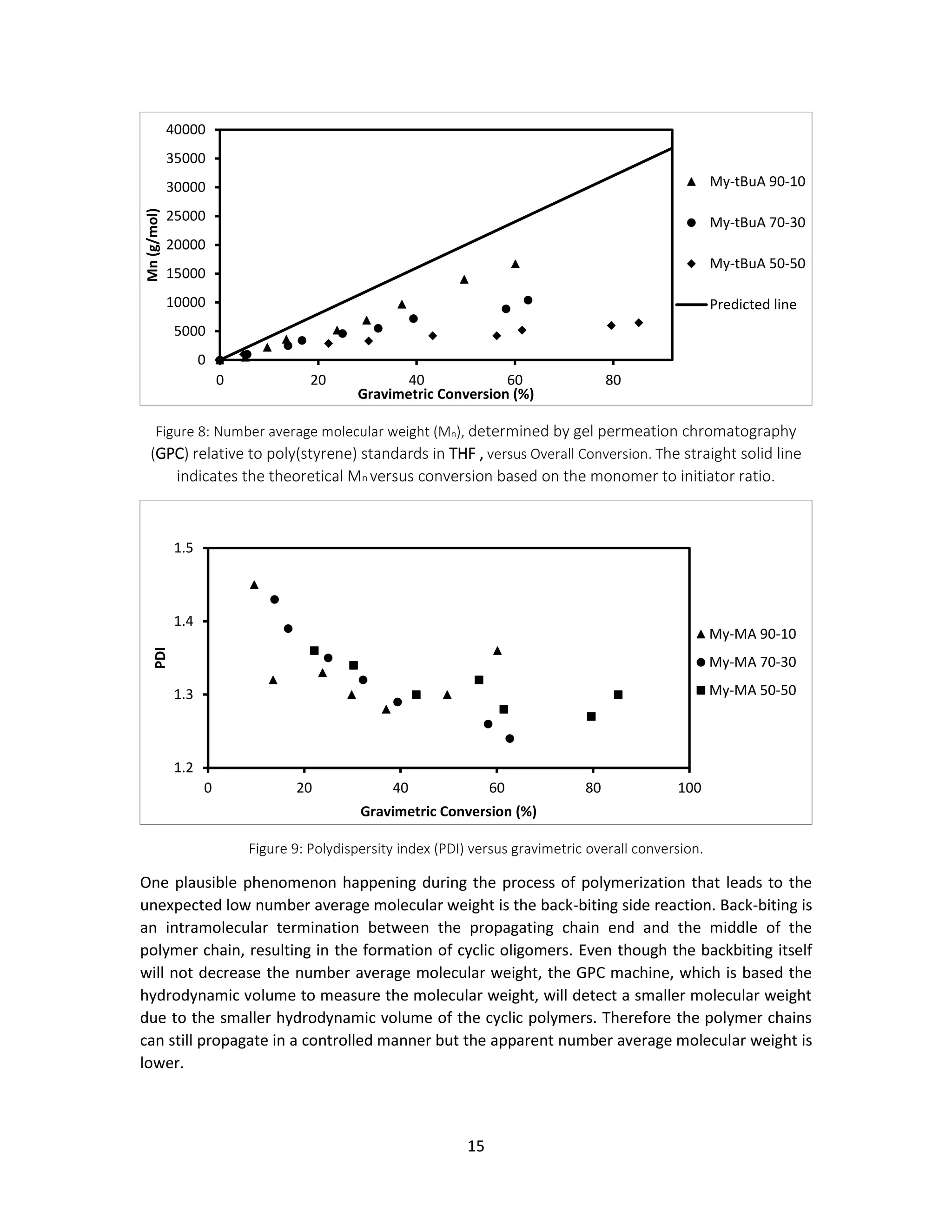
Figure 8 illustrates the number average molecular weight versus gravimetric conversion for
different initial feed compositions of myrcene (fmyrcene0). Here gravimetric conversion is used
instead of overall conversion because the peaks for the chemical shift of 5.12ppm on myrcene in
1
H NMR were failed to be detected, and therefore the conversions based on NMR are not
applicable. It is noteworthy that as fmyrcene decreases, the number average molecular weight
consistently decreases. Normally, such deviations from the predicted line indicates that the
polymerizations are not well-controlled. However, according to Figure 9, the polydispersity
indexes of the processes are all below 1.5 and trending to become steadier, which obeys the
characteristic of controlled polymerization. Thus some other factors bias the number average
molecular weight although the polymerization is well-controlled.
0
5000
10000
15000
20000
25000
30000
35000
40000
0 20 40 60 80
Mn(g/mol)
Overall conversion (%)
Experimental Mn versus conversion
Corrected Mn versus conversion
Predicted line](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-14-2048.jpg)
![16
Several publications have shown that back-biting reactions happen frequently when tert-butyl
acrylate is involved in some anionic polymerizations and controlled radical polymerizations[11], [12],
[13]
. The rate of back-biting is a strong function of temperature, and can increase 7-fold more
when the temperature goes from 20o
C to 80o
C[13]
. Therefore, at an experimental temperature of
115 o
C, there is a high possibility that the back-biting will happen. Besides, the back-biting in
block copolymerization of methyl methacrylate and tert-butyl acrylate shows the same
characteristics on the results (low Mn, low PDI)[11]
, which proves the above hypothesis. However,
since tert-butyl-acrylate is often used for block copolymerization instead of random or
alternating copolymerization, some further experiments are required to sufficiently confirm the
hypothesis of the existence of back-biting in myrcene/tert-butyl acrylate copolymerization.](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-16-2048.jpg)

![18
6. References
[1] A. Behr and L. Johnen, “Myrcene as a Natural Base Chemical in Sustainable Chemistry: A
Critical Review,” ChemSusChem, vol. 2, no. 12, pp. 1072–1095, Dec. 2009.
[2] J. M. Bolton, M. A. Hillmyer, and T. R. Hoye, “Sustainable thermoplastic elastomers from
terpene-derived monomers,” ACS Macro Lett., vol. 3, no. 8, pp. 717–720, 2014.
[3] K. Satoh, A. Nakahara, K. Mukunoki, H. Sugiyama, H. Saito, and M. Kamigaito,
“Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: living
cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-
weight hydrogenated poly(β-pinene),” Polym. Chem., vol. 5, no. 9, pp. 3222–3230, 2014.
[4] R. J. Young and P. A. Lovell, Introduction to Polymers, 3rd ed. Taylor & Francis Group, LLC,
2002.
[5] B. H. Lessard, “Random controlled free radical copolymeriza2on of acrylic acid / styrene
and tert- -‐ butyl acrylate / styrene mixtures using nitroxide mediators By,” 2008.
[6] C. Zhang and M. Maric, “Synthesis of Stimuli-responsive, Water-soluble Poly[2-
(dimethylamino)ethyl methacrylate/styrene] Statistical Copolymers by Nitroxide
Mediated Polymerization,” Polymers (Basel)., vol. 3, no. 3, pp. 1398–1422, 2011.
[7] F. Fringuelli and A. Taticchi, The Diels–Alder Reaction. 2001.
[8] A. Tsujii, M. Namba, H. Okamura, and A. Matsumoto, “Radical alternating
copolymerization of twisted 1,3-butadienes with maleic anhydride as a new approach for
degradable thermosetting resin,” Macromolecules, vol. 47, no. 19, pp. 6619–6626, 2014.
[9] G. C. Alexander and M. E. Paulaitis, “Comment on ‘Kinetics of a Diels-Alder Reaction of
Maleic Anhydride and Isoprene in Supercritical CO2,’” J. Phys. Chem. A, vol. 107, no. 43,
pp. 9248–9249, 2003.
[10] E. Ceausescu, Stereospecific polymerization of isoprene. Pergamon Press, 1983.
[11] E. Čadová, J. Dybal, J. Kříž, P. Vlček, M. Janata, and L. Toman, “Back-Biting Termination in
Methyl Methacrylate/ tert- Butyl Acrylate Anionic Block Copolymerization,” Macromol.
Chem. Phys., vol. 209, no. 16, pp. 1657–1665, 2008.
[12] T. Ishizone, K. Yoshimura, A. Hirao, and S. Nakahama, “Controlled Anionic Polymerization
of tert -Butyl Acrylate with Diphenylmethyl Anions in the Presence of Dialkylzinc,”
Macromolecules, vol. 31, no. 25, pp. 8706–8712, 1998.
[13] B. Wenn and T. Junkers, “Kilohertz Pulsed-Laser-Polymerization : Simultaneous
Determination of Backbiting , Secondary , and Tertiary Radical Propagation Rate
Coefficients for tert -Butyl Acrylate,” pp. 1–7, 2016.](https://image.slidesharecdn.com/49072bc5-0cdb-4522-b853-65d170457f92-160520182827/75/Lab-report-18-2048.jpg)