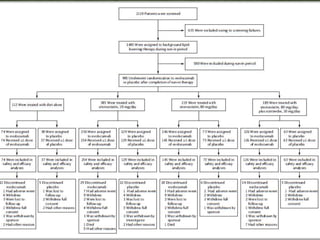
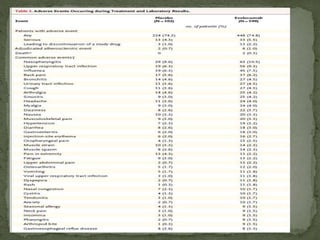
This phase 3 clinical trial evaluated the safety and efficacy of evolocumab, a monoclonal antibody that inhibits PCSK9, in over 900 patients with hyperlipidemia over 52 weeks. Patients received evolocumab or placebo injections monthly in addition to background lipid-lowering therapy. The primary outcome was the percent change in LDL cholesterol from baseline to week 52. Evolocumab reduced LDL cholesterol by 57% on average compared to placebo and over 80% of patients achieved an LDL cholesterol level under 70 mg/dL. More adverse events occurred in the evolocumab group, including myalgia and elevated creatine kinase. The study demonstrated evolocumab significantly lowered LDL cholesterol over 52 weeks when added to standard lipid-lower