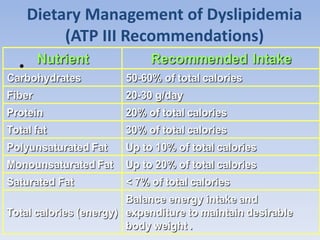
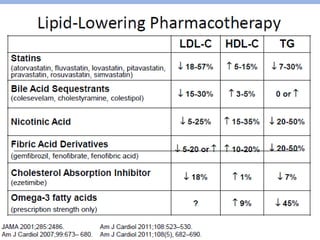
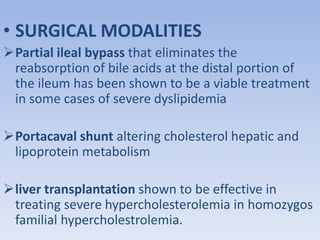
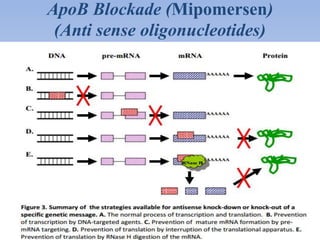
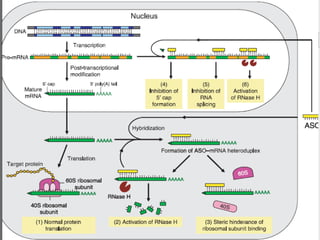
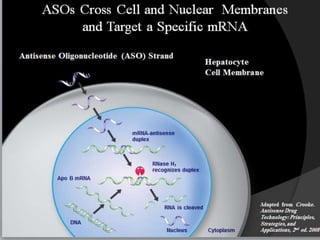
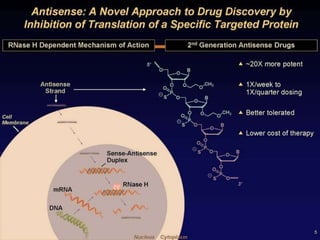
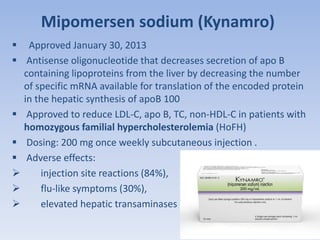
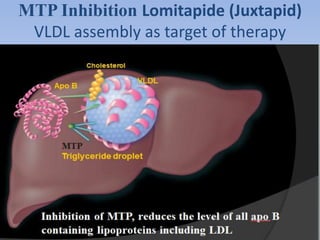
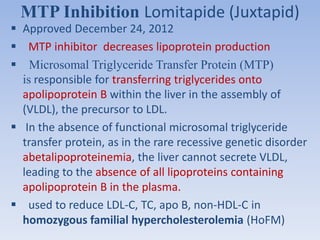
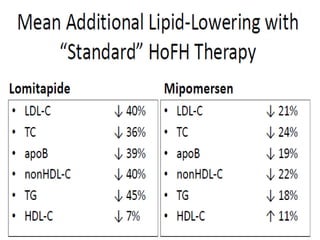
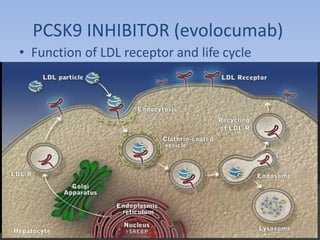
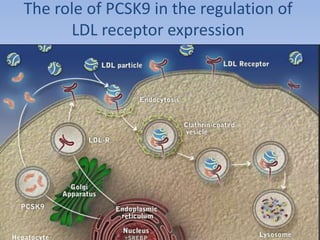
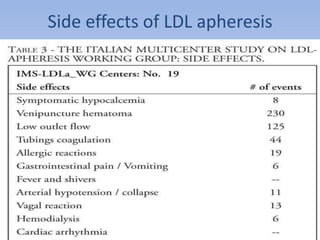
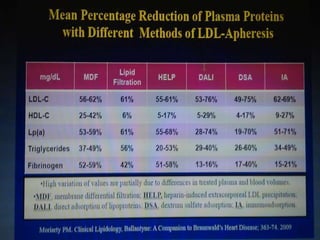
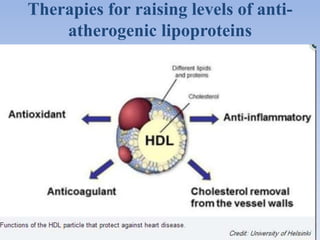
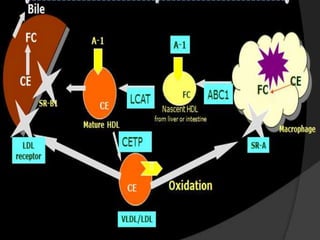
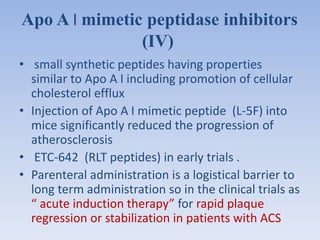
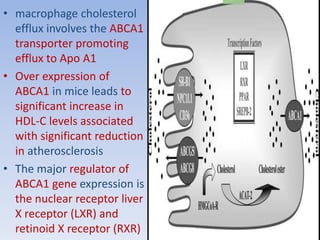
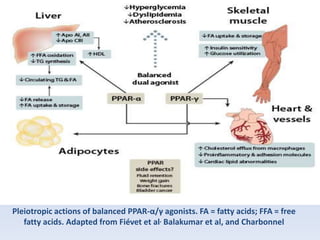
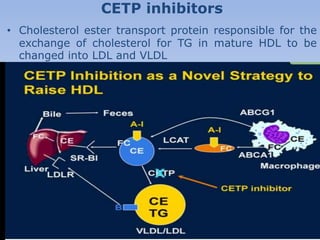
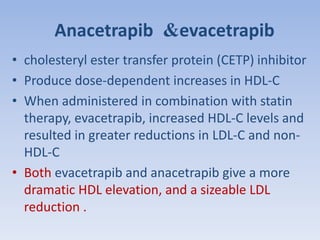
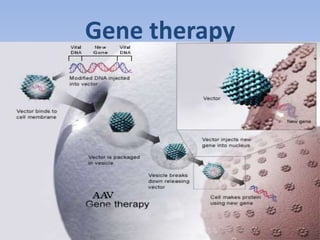
This document summarizes recent therapeutic approaches for dyslipidemia. It discusses lifestyle changes like smoking cessation, weight loss, improved diet, and increased physical activity. It also discusses pharmacological management including ATP III guidelines, novel therapies that block lipoprotein output or increase clearance like PCSK9 inhibitors. Therapies to raise anti-atherogenic lipoproteins by promoting ApoA1 production or cholesterol efflux are also covered, as well as therapies slowing HDL removal and dual PPAR agonists. Surgical modalities like partial ileal bypass and liver transplantation are mentioned for severe dyslipidemia cases.