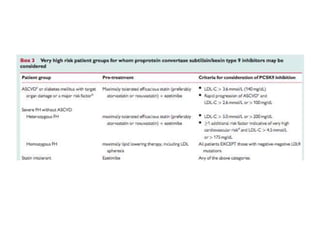
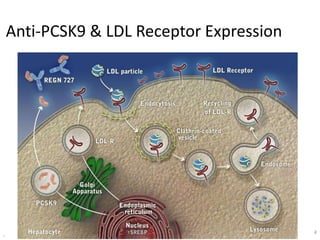
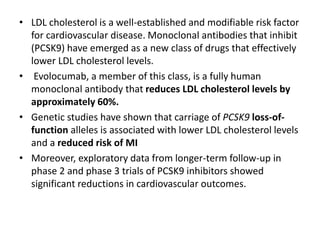
- Evolocumab is a fully human monoclonal antibody that reduces LDL cholesterol levels by approximately 60% when used in addition to statin therapy.
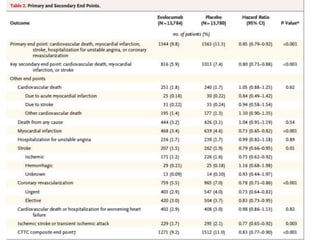
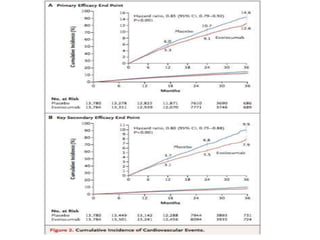
- A large clinical trial found that evolocumab significantly reduced the risk of cardiovascular events such as heart attack and stroke in patients with cardiovascular disease. Evolocumab lowered LDL cholesterol levels to less than 70 mg/dL in 87% of patients.
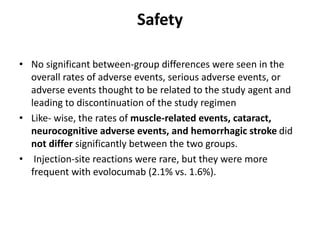
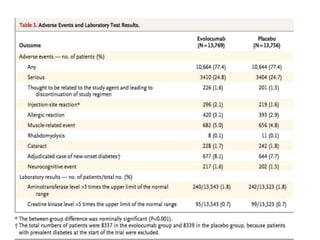
- The benefits of evolocumab in reducing cardiovascular outcomes were consistent across various patient groups and baseline LDL cholesterol levels and were maintained over time with treatment. No significant safety issues were identified with evolocumab.
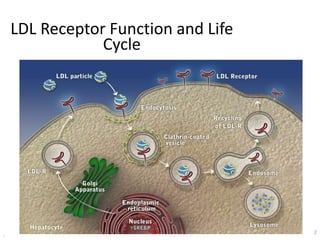
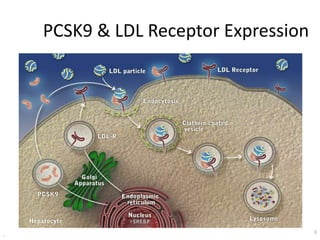




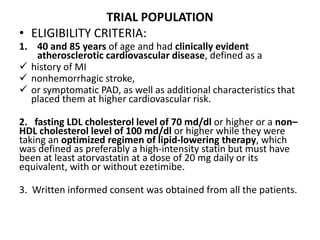
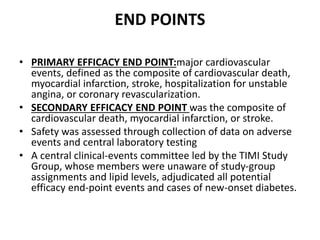
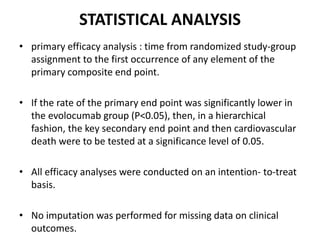

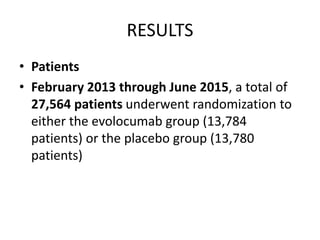
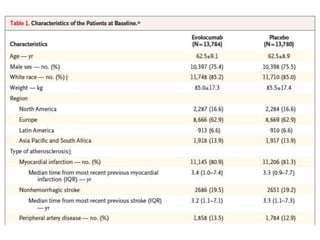
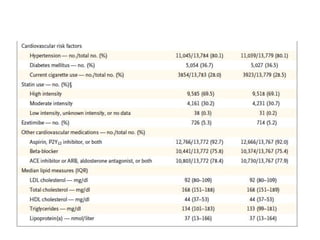

![At 48 weeks, the least-squares mean percentage reduction in LDL cholesterol levels with
evolocumab, as compared with placebo, was 59% (95% confidence interval [CI], 58 to 60;
P<0.001), for a mean absolute reduction of 56 mg per deciliter (95% CI, 55 to 57) to a median of
30 mg per deciliter (interquartile range, 19 to 46) .The reduction in LDL cholesterol levels was
maintained over time](https://image.slidesharecdn.com/jcwednesday29thmarch2017-170522005041/85/PCKS9-INHIBITORS-21-320.jpg)