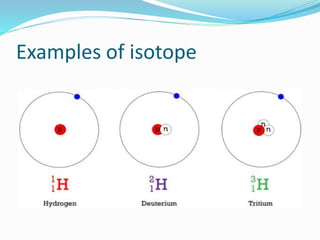
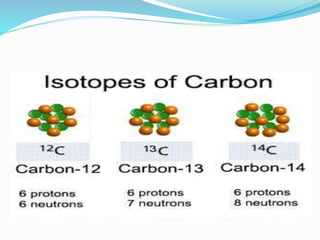
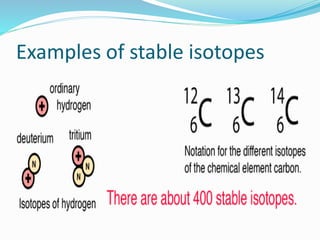
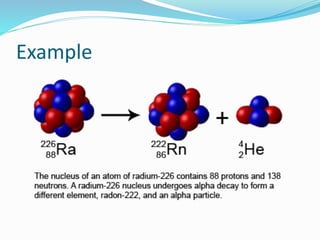
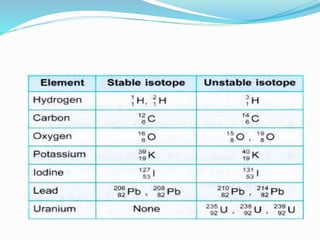
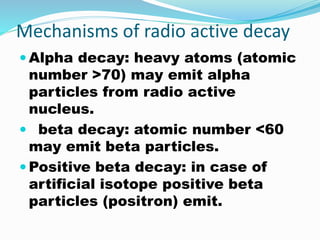
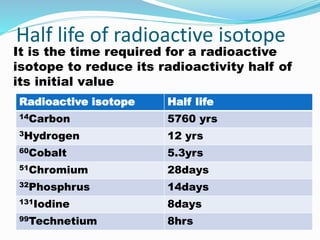
Isotopes are variants of elements with the same number of protons but different numbers of neutrons, resulting in different atomic weights. They can be stable or unstable, with unstable isotopes undergoing radioactive decay to achieve stability, emitting radiation in the process. Radioactive isotopes have significant clinical applications, such as in diagnostics and therapy, but also pose hazards including immediate health effects and long-term risks like carcinogenesis.