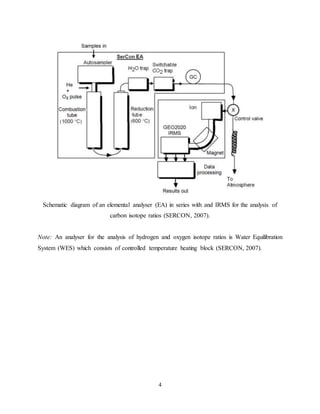
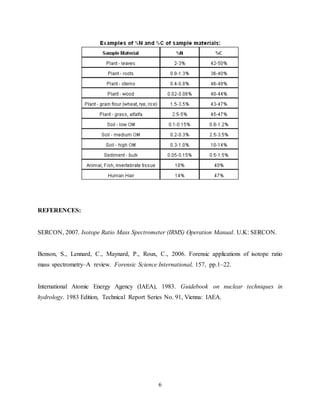
The document discusses isotope ratio mass spectrometry (IRMS) and its application in stable isotope analysis. IRMS allows for precise measurements of stable isotope ratios in samples to determine their composition and origin. It involves reducing complex compounds in a sample to simple molecules like CO2 or N2, then using a mass spectrometer to separate the different isotopes based on their mass and measure their ratios. This provides information about isotopes like hydrogen, oxygen, carbon, nitrogen and sulfur in the sample. The document also describes the principles and procedures for analyzing specific isotopes like carbon-13, nitrogen-15, hydrogen-2 and oxygen-18 using a continuous flow isotope ratio mass spectrometer.