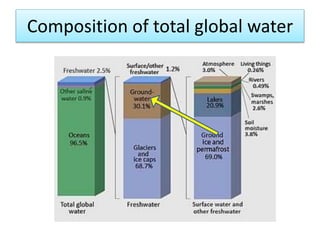
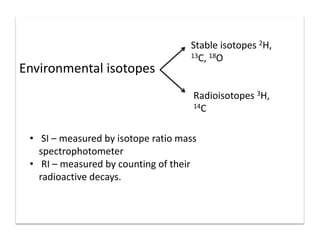
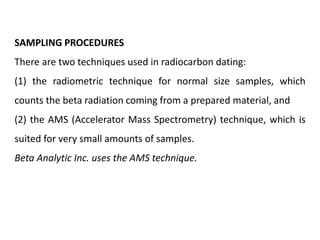
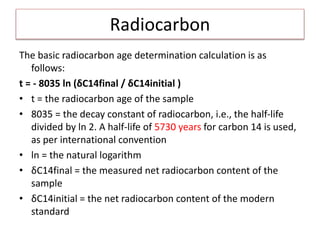
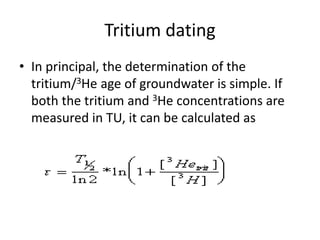
This document discusses the use of radioisotopes in groundwater research. It begins by providing background on groundwater and then discusses why further research is needed due to poor understanding and management of groundwater resources. It describes how stable and radioactive isotopes can be effective tools for hydrological investigations by helping to study recharge sources and rates, groundwater ages, aquifer interactions, and groundwater quality issues like salinization and pollution. Specific isotopes discussed include radiocarbon, tritium, and environmental isotopes. Applications and current uses in developing countries are also summarized.