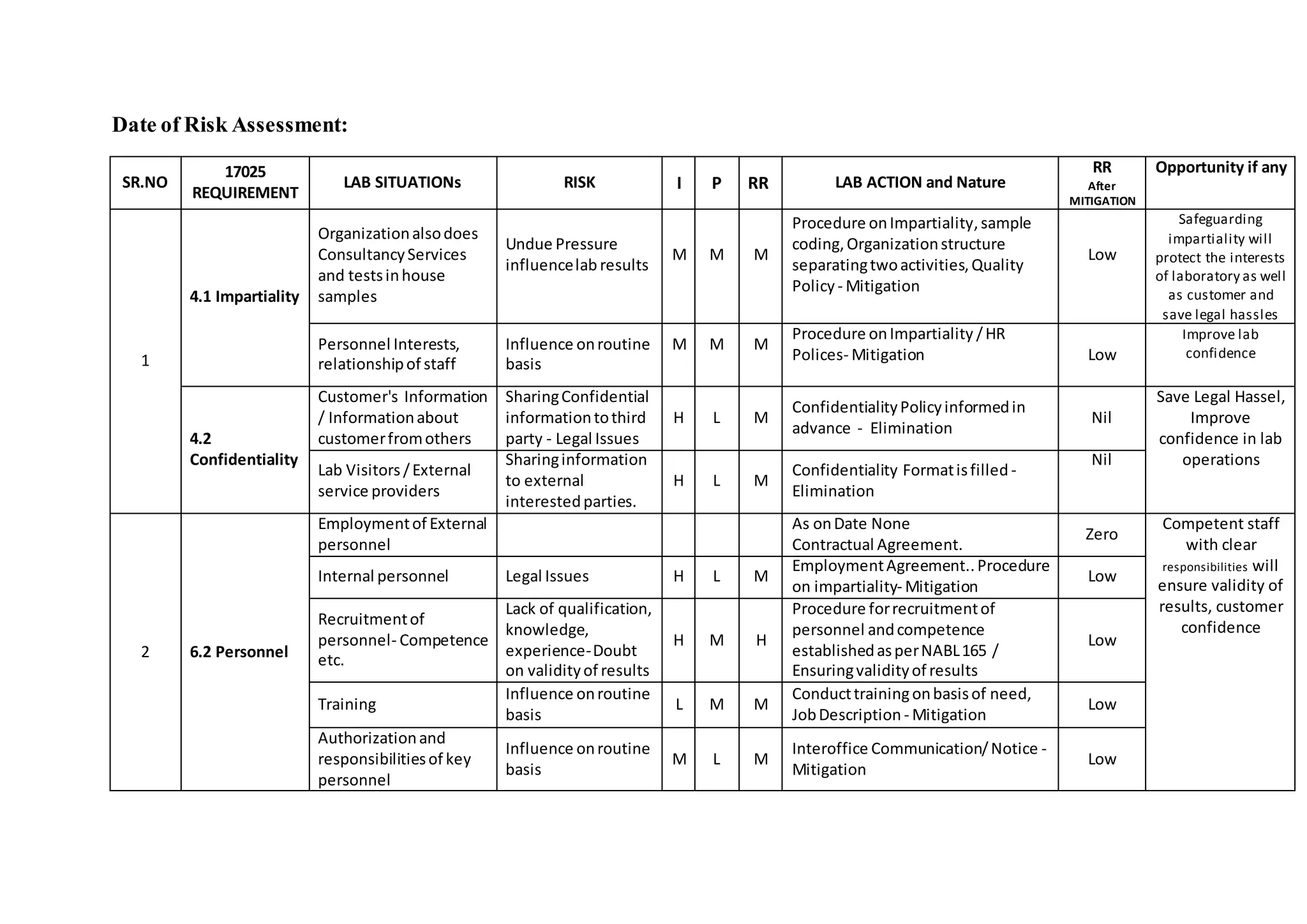
This document summarizes the risks identified in complying with ISO/IEC 17025 requirements and the actions taken by the laboratory to mitigate those risks. It identifies 14 main risk areas related to impartiality, confidentiality, personnel, facilities, equipment, purchasing, methods, sampling, test items, measurement uncertainty, validity of results, reporting, complaints, and management system documentation. For each risk, it evaluates the impact and likelihood, identifies mitigation actions taken through procedures, policies, and controls, and reduces the residual risk. The overall goal is to ensure the validity of test results, protect confidentiality, maintain impartiality, and improve customer satisfaction and confidence in the laboratory's operations.