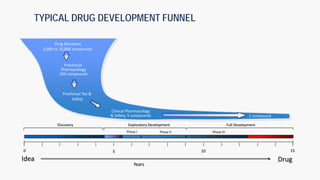
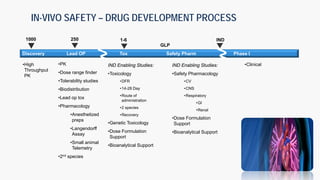
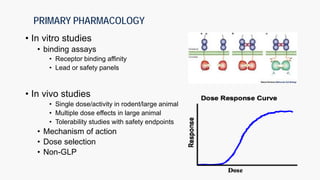
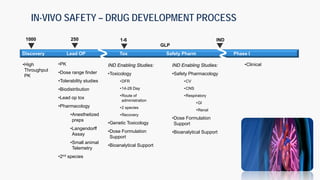
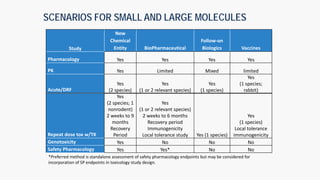
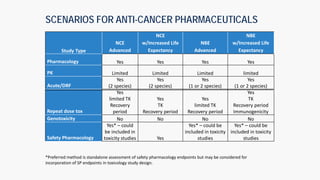
The document discusses the drug development process from pre-clinical research through Investigational New Drug (IND) application. It outlines the typical studies required to evaluate safety and toxicity, identify target organs of toxicity, determine appropriate dosing for clinical trials, and communicate risks. These include pharmacology, pharmacokinetics, safety pharmacology, acute and repeat-dose toxicology studies in two animal species along with genetic toxicology assays. The goals are to estimate initial safe doses for clinical trials and identify parameters that can monitor toxicity. The process seeks to identify potentially safe compounds for human testing while eliminating those that may be unsafe.