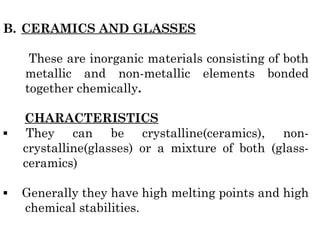
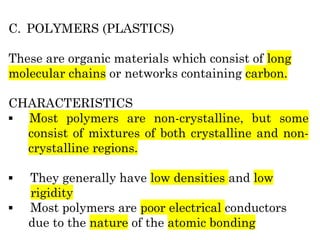
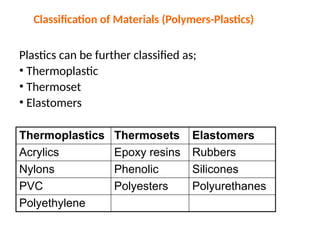
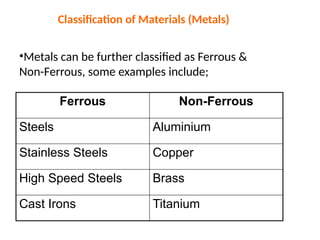
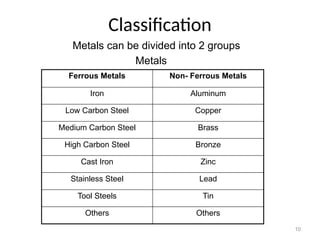
The document provides an overview of the classification of engineering materials, detailing four main categories: metals and alloys, ceramics, polymers, and composites. It elaborates on the properties and classifications of metals, including ferrous and non-ferrous types, as well as various types of steel. Additionally, it discusses ceramics, polymers, composite materials, and smart materials, highlighting their characteristics and applications in engineering and design.



![1.1 CLASSIFICATION OF ENGINEERING
MATERIALS
METALS AND ALLOYS
These are inorganic materials composed of one or
more metallic elements
Characteristics
▪ They usually have a crystalline structure and are good
thermal and electrical conductors
▪ Many metals have high strength and high elastic moduli
[changes its shape only slightly under elastic loads (e.g. diamond))]
▪ They maintain their good strength at high and low
temperatures.](https://image.slidesharecdn.com/engineeringmaterialsandtheirproperties-240819173341-4b85b21f/85/Engineering-Materials-and-their-properties-pptx-4-320.jpg)









![16
Cast Iron
Contains 2%-4% of carbon
Very hard and brittle
Strong under compression
Suitable for casting [can be poured at a relatively
low temperature]
Examples are:
Engine block, engineer vices, machine parts](https://image.slidesharecdn.com/engineeringmaterialsandtheirproperties-240819173341-4b85b21f/85/Engineering-Materials-and-their-properties-pptx-16-320.jpg)