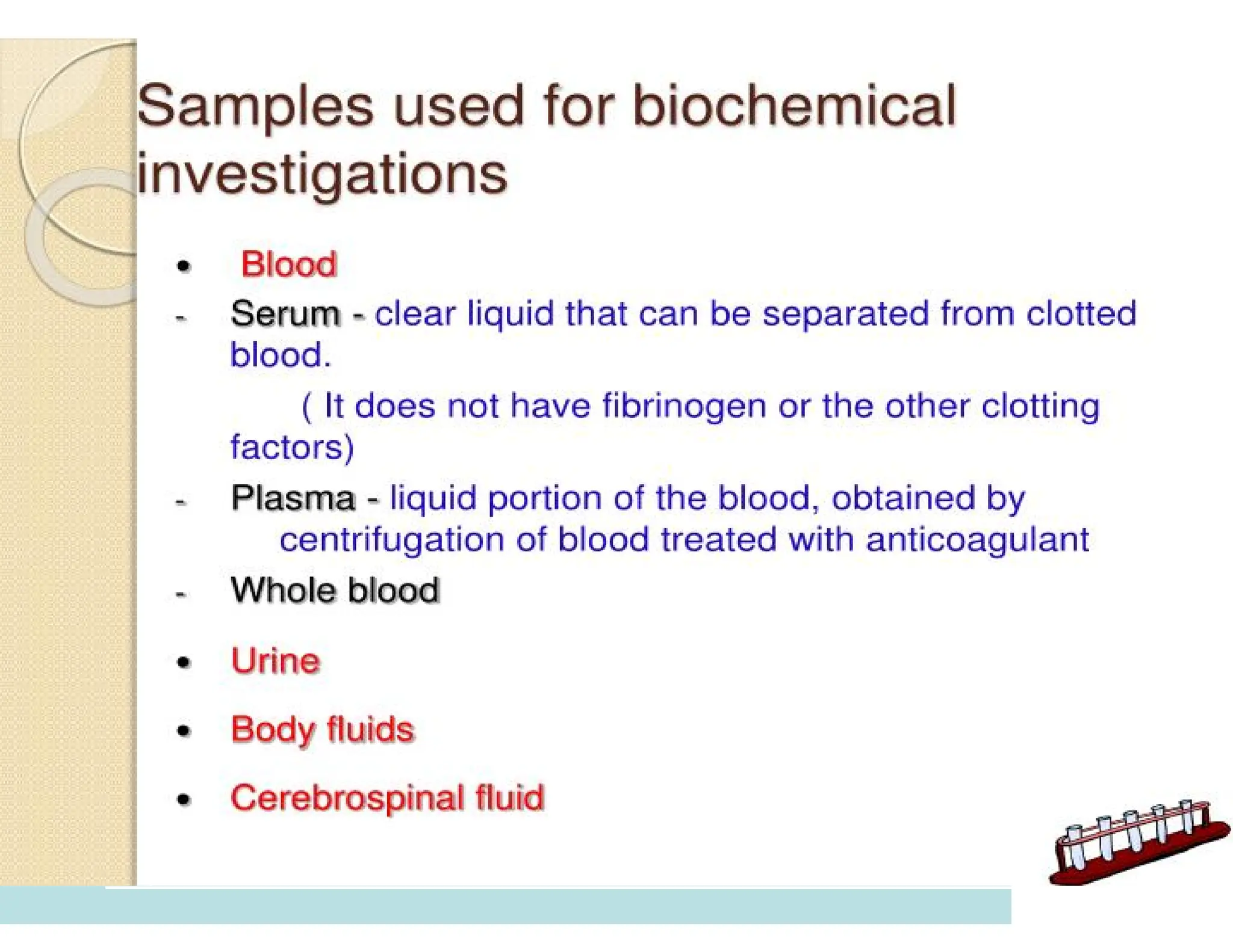
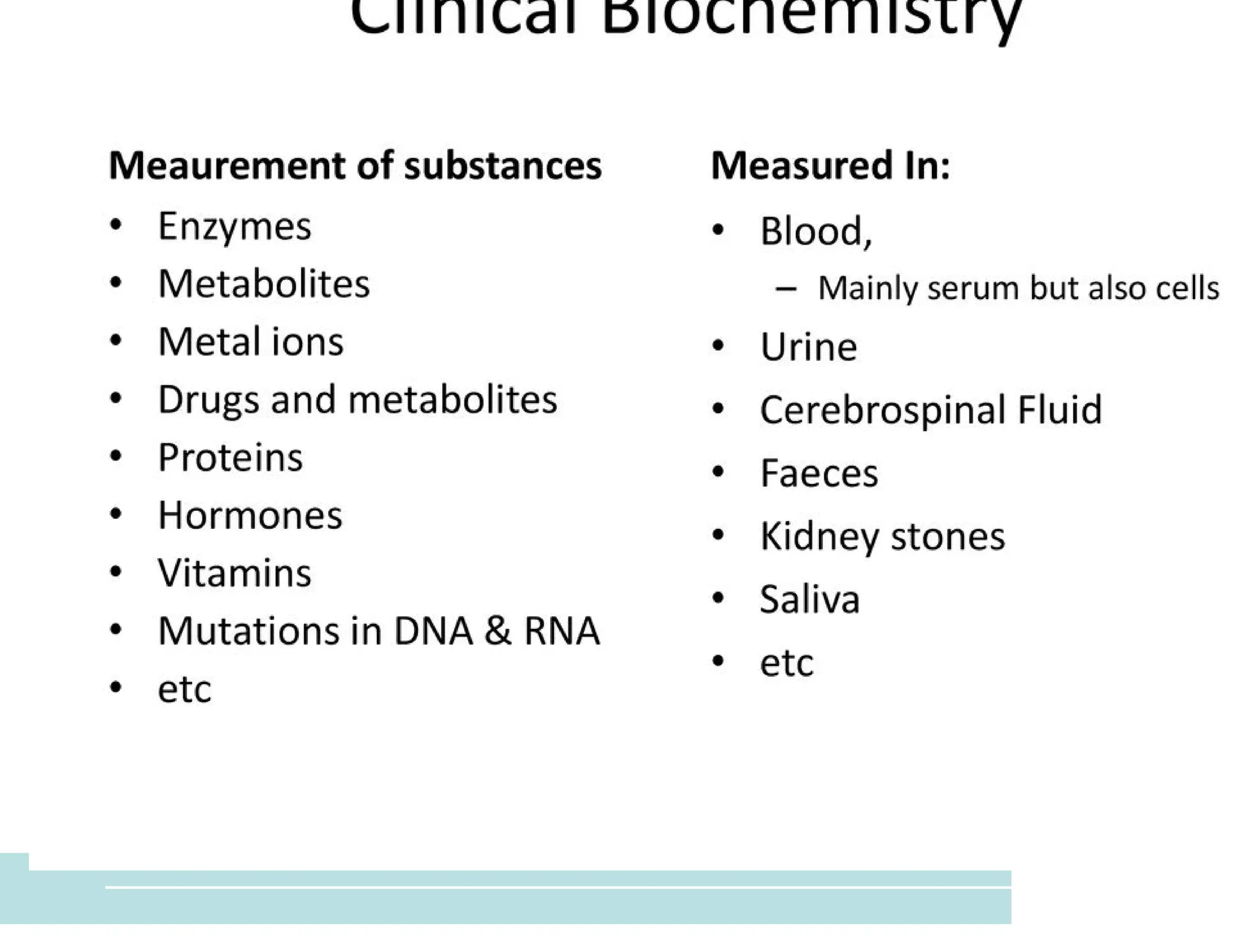
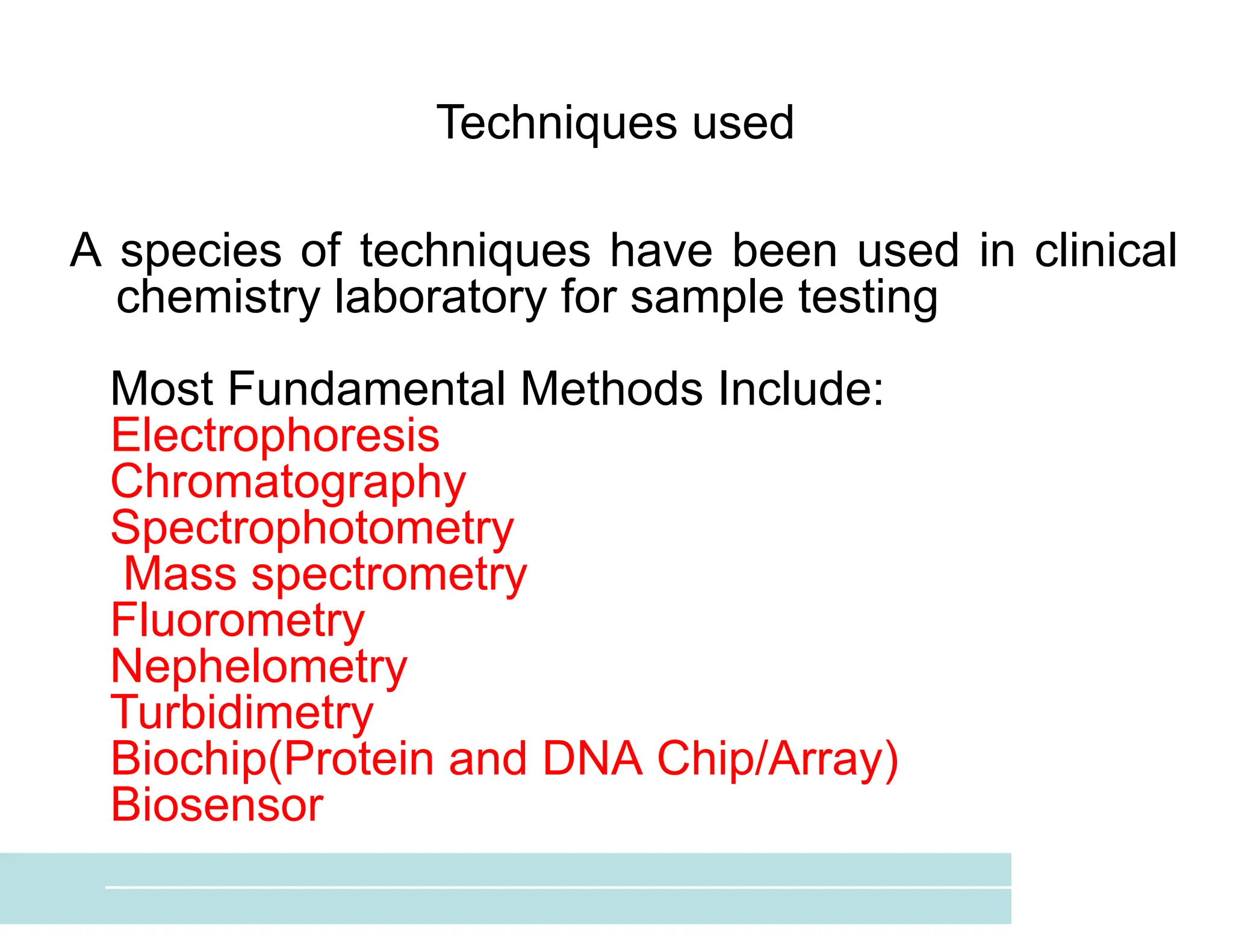
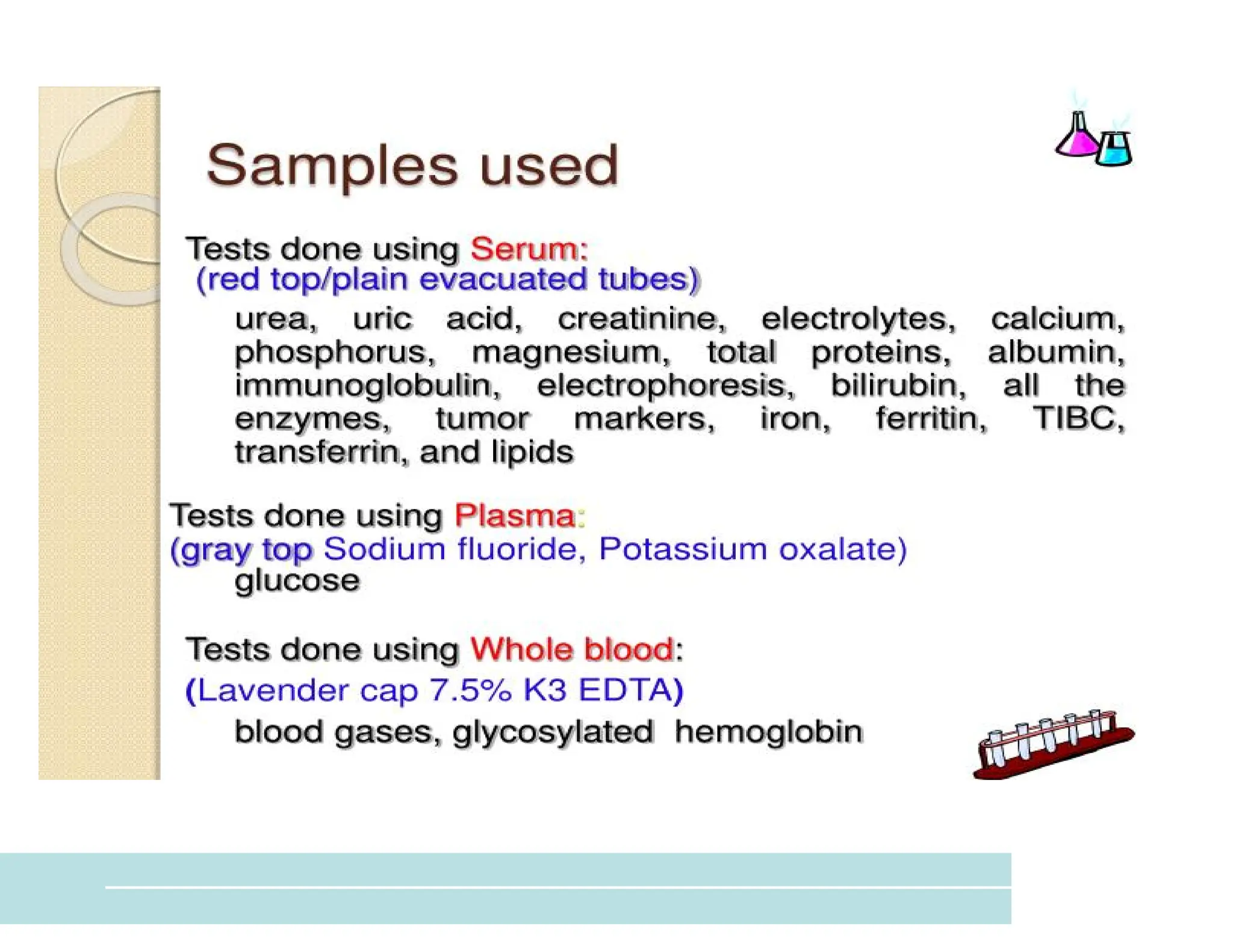
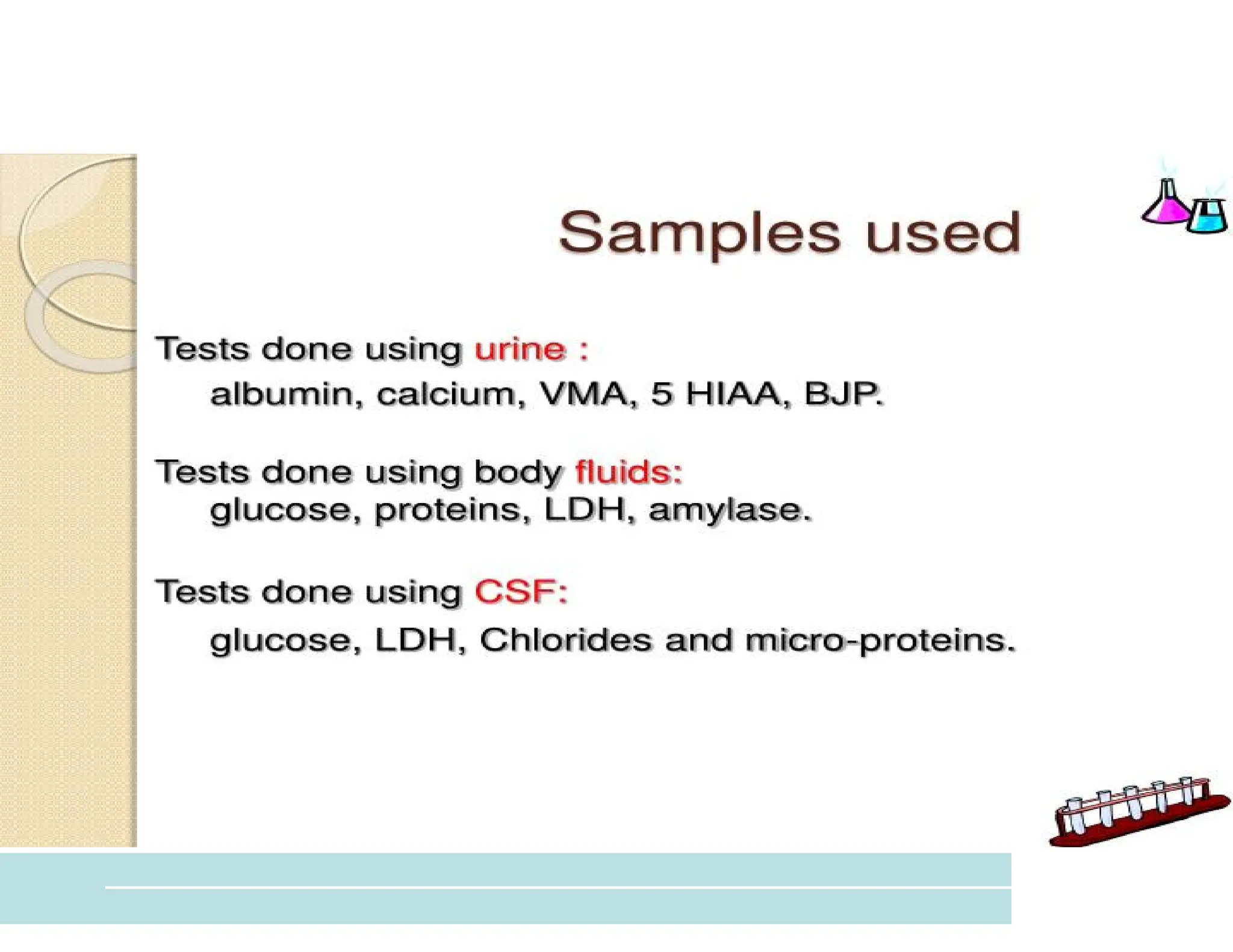
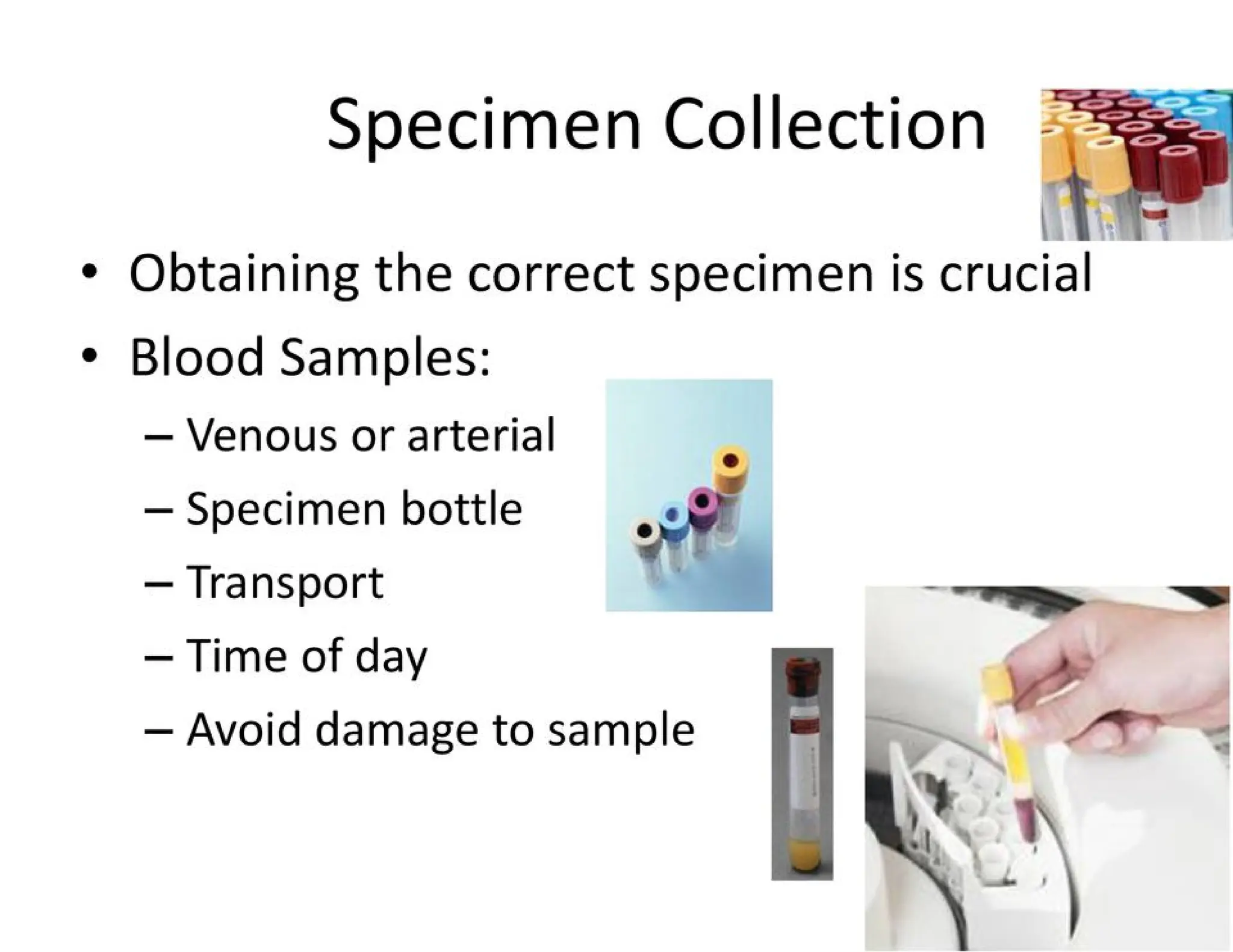
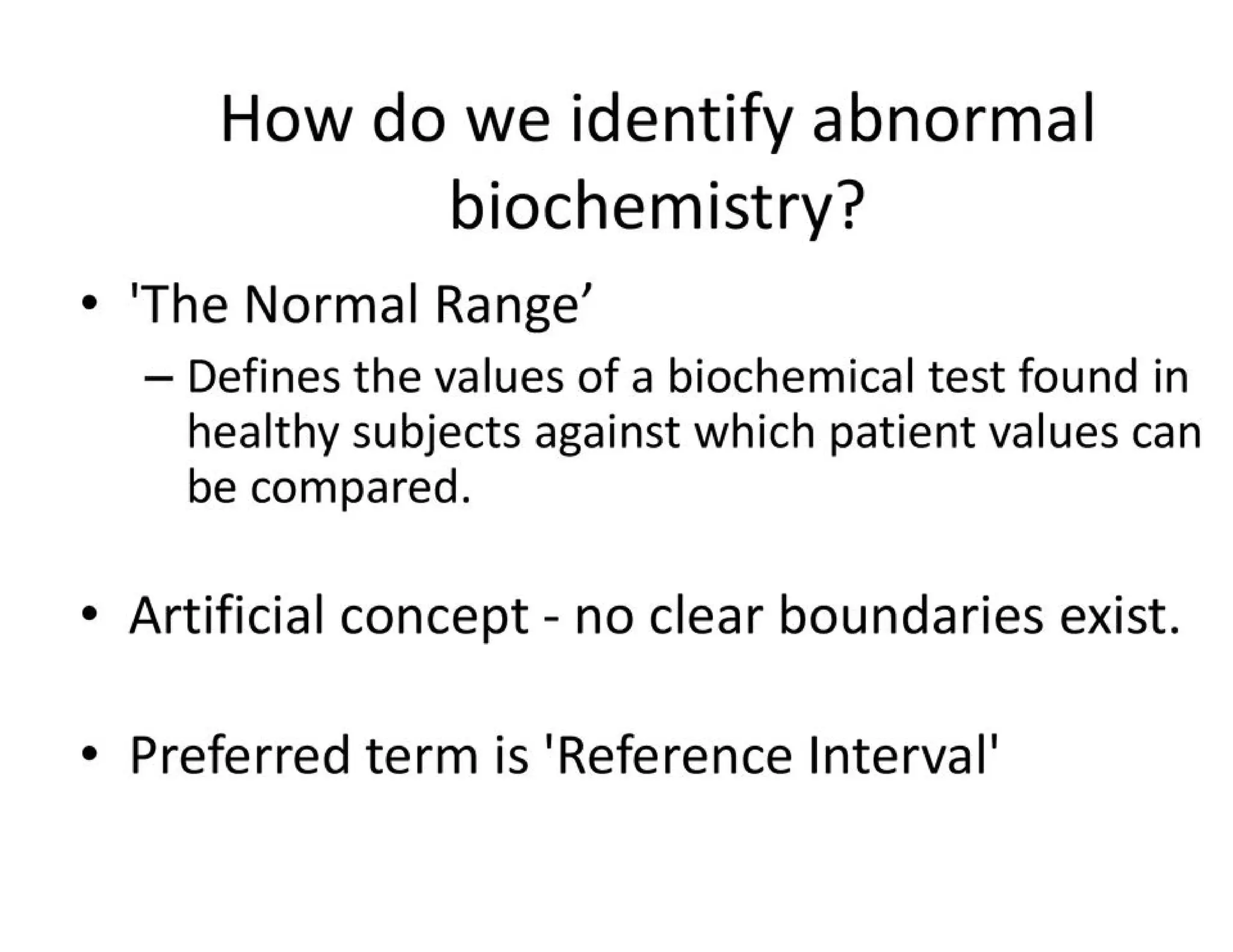
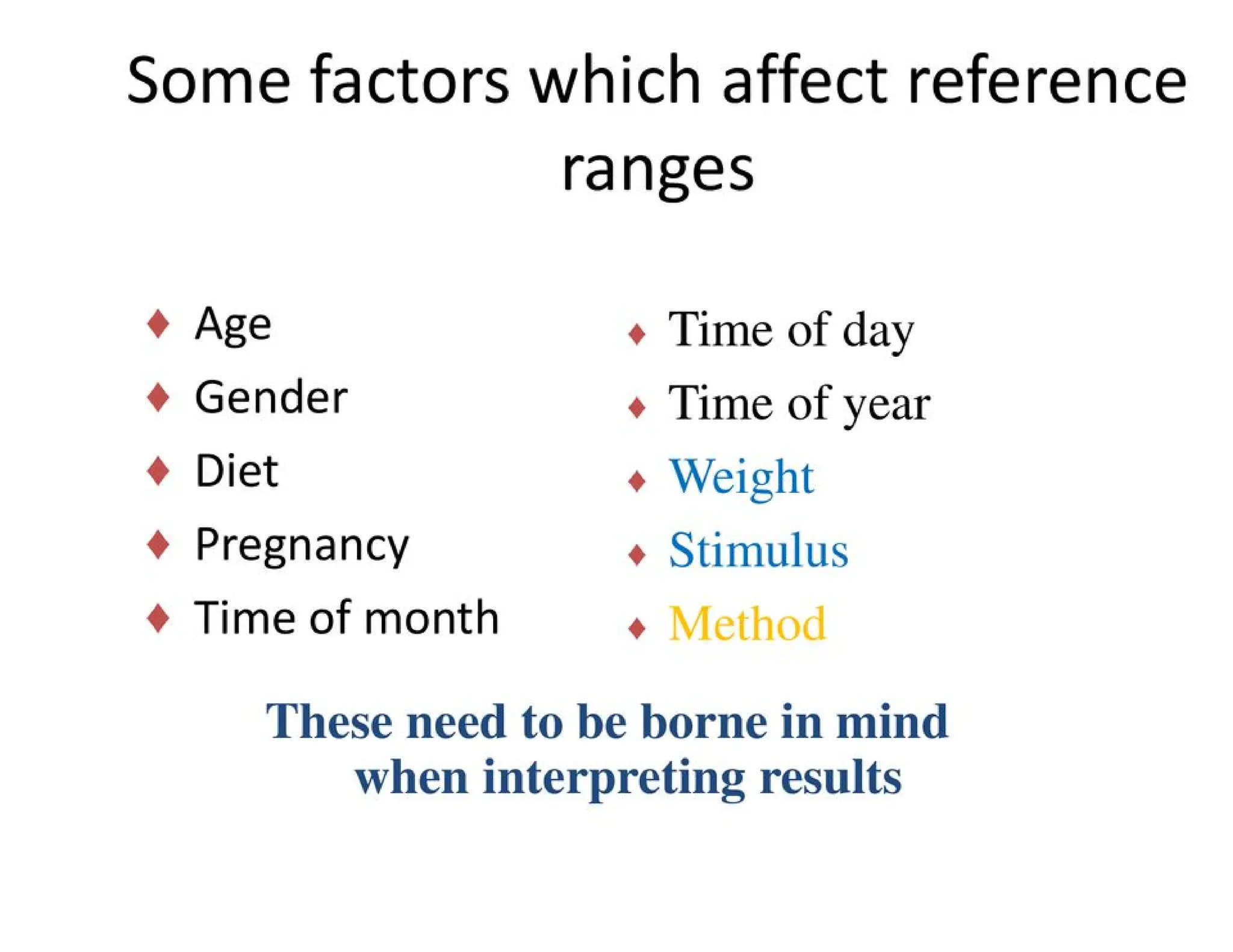
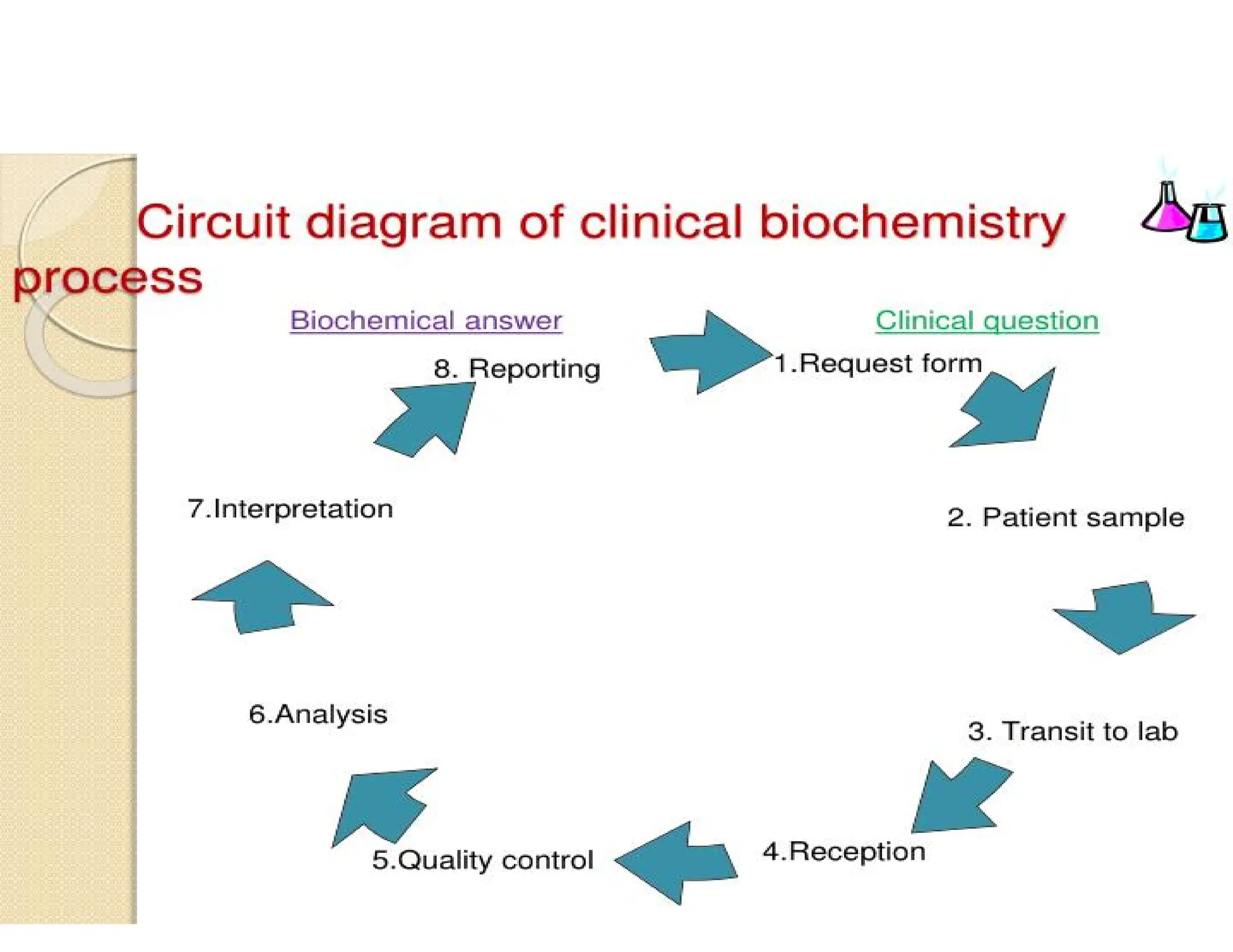
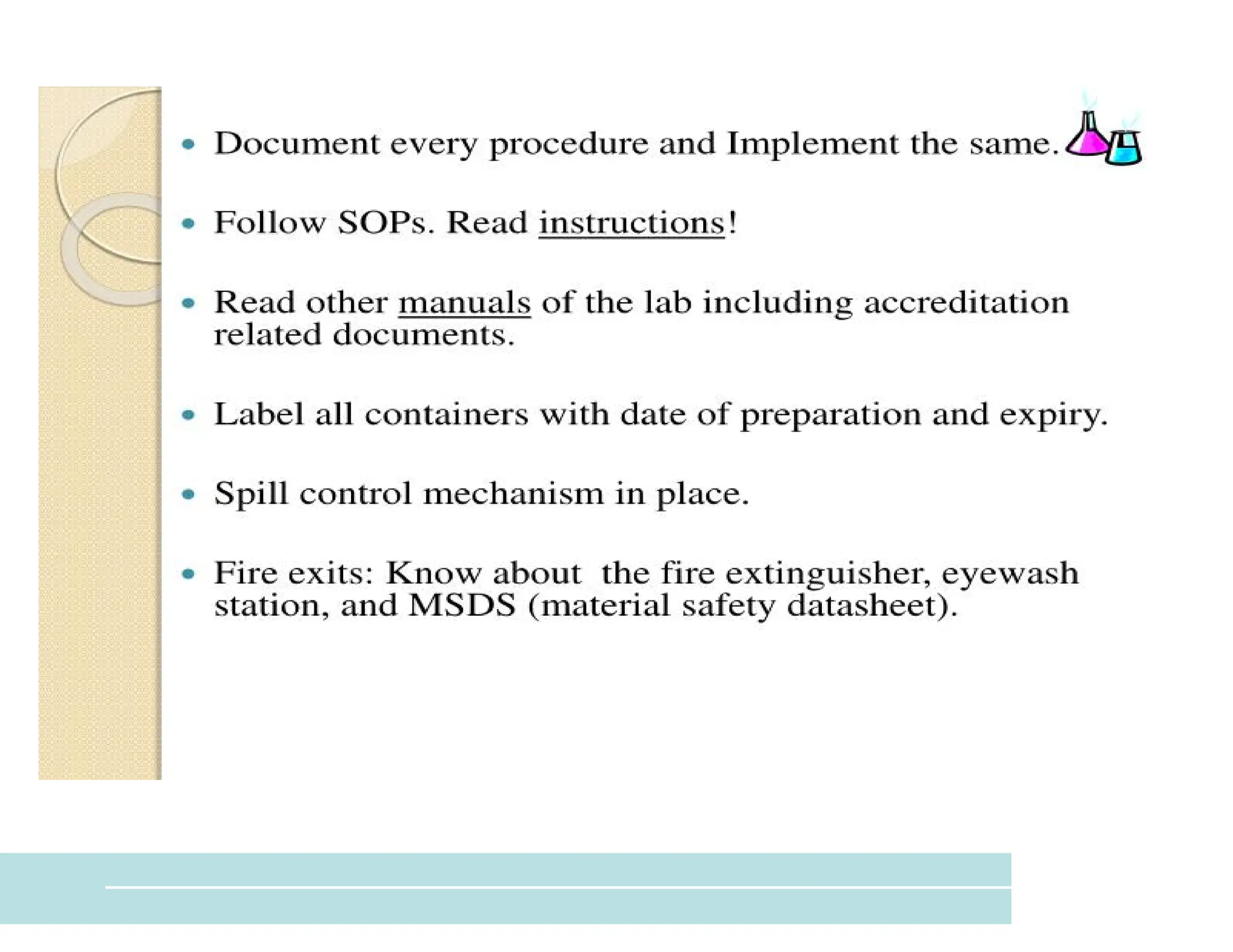
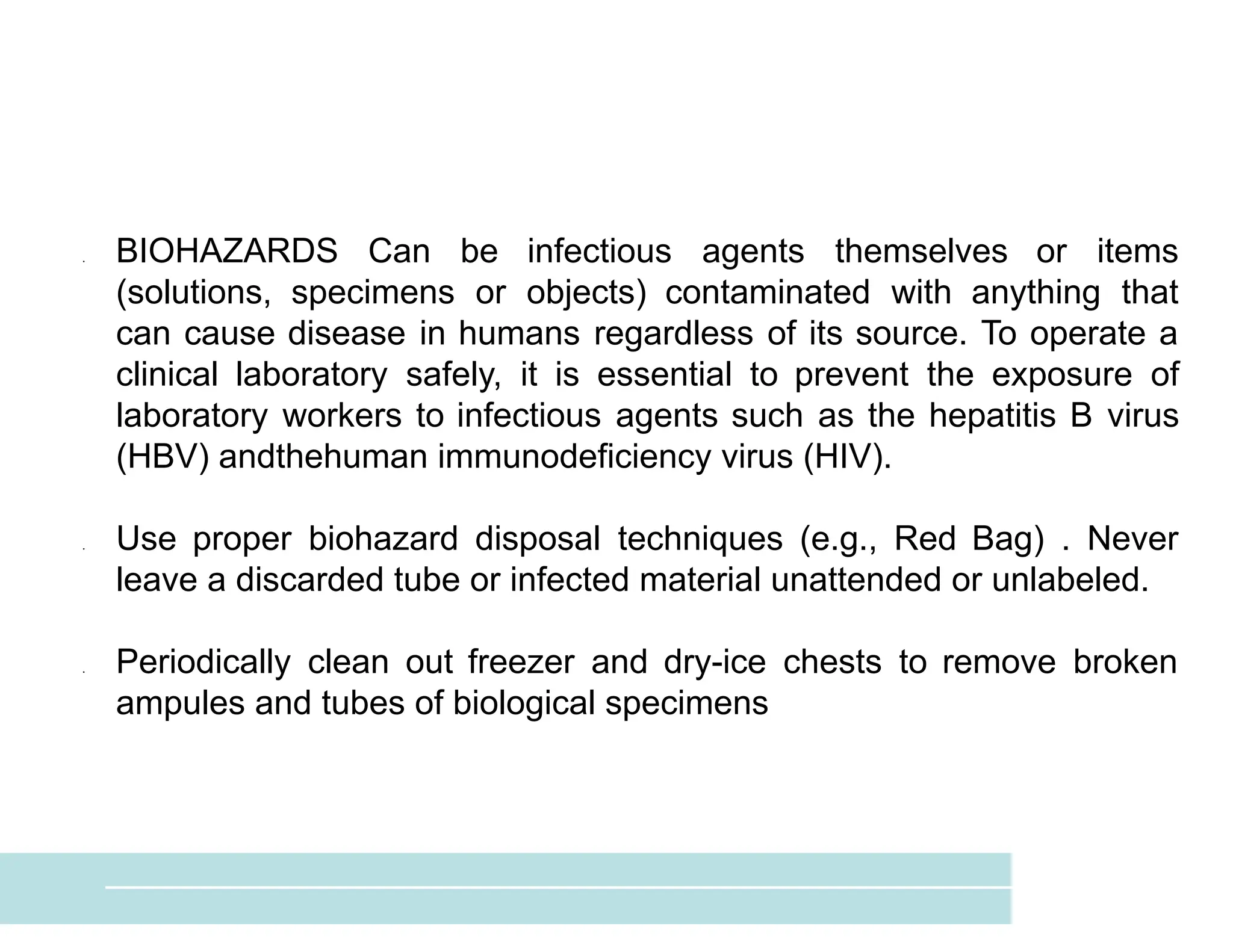
The document provides an overview of biochemistry and its application in clinical settings, highlighting its role in diagnosing and monitoring diseases through the analysis of biochemical markers in body fluids. It emphasizes the importance of safety in clinical laboratories, detailing various hazards and safety measures associated with chemical, electrical, and biological risks. Additionally, it covers techniques used in clinical chemistry for testing, underscoring the advancements in technology that improve diagnostic accuracy.