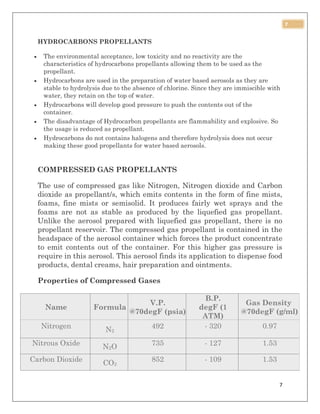
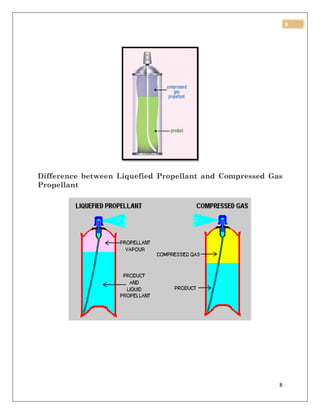
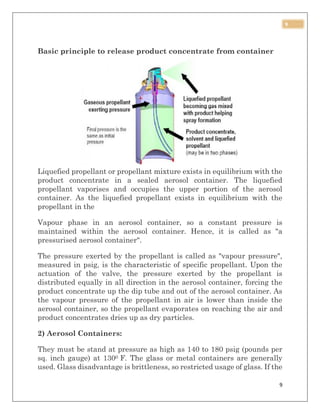
This document discusses aerosol packaging and summarizes the key components. It acknowledges those who provided guidance for the project. It then introduces aerosol sprays, noting they create a mist from a pressurized can. The document defines an aerosol and lists advantages such as targeted dosing and avoiding degradation. It also outlines disadvantages like cost and potential irritancy. The main components of an aerosol package are identified as the propellant, container, and valve/actuator. Different types of propellants are described including CFCs, HCFCs, HFCs, hydrocarbons, and compressed gases.