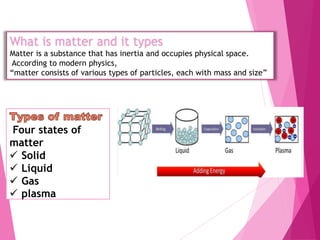
The document discusses plasma, the fourth state of matter. It provides an overview of plasma, including its history, properties, forms, locations, types and applications. Some key points covered are:
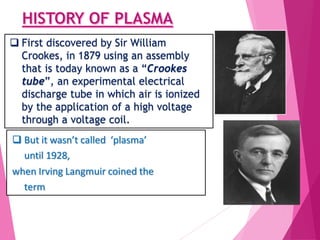
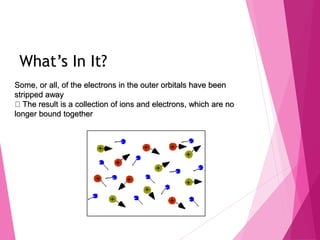
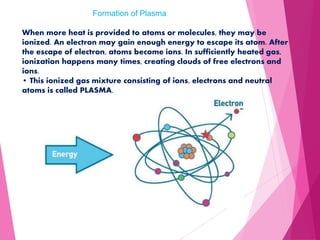
- Plasma was first discovered in 1879 but was coined the term in 1928. It is an ionized gas consisting of free electrons and ions.
- Plasma exists in both natural forms like lightning and stars, and artificial forms like neon signs and fluorescent lights.
- There are different types of plasma including cold, hot, and ultracold plasma.
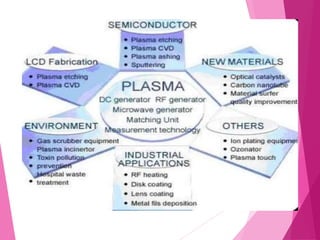
- Applications of plasma include lighting, semiconductor manufacturing, displays, welding, cleaning, and fusion power research.