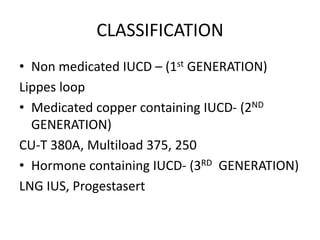
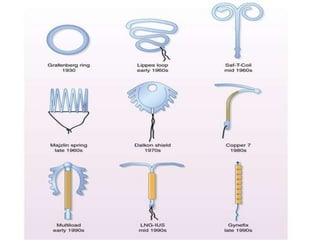
1. Intrauterine contraceptive devices (IUCDs) include non-medicated, copper-containing, and hormone-containing devices.
2. Copper IUCDs like CU-T 380A release copper ions that create an inflammatory response and changes in the endometrium to prevent implantation. The levonorgestrel IUCD releases the hormone progestin to suppress the endometrium.
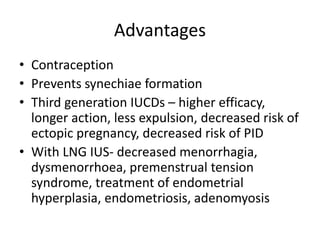
3. IUCDs are highly effective long-acting reversible contraceptives that also provide non-contraceptive benefits like lighter periods. Their failure rates are much lower than other methods. Complications are generally minor.