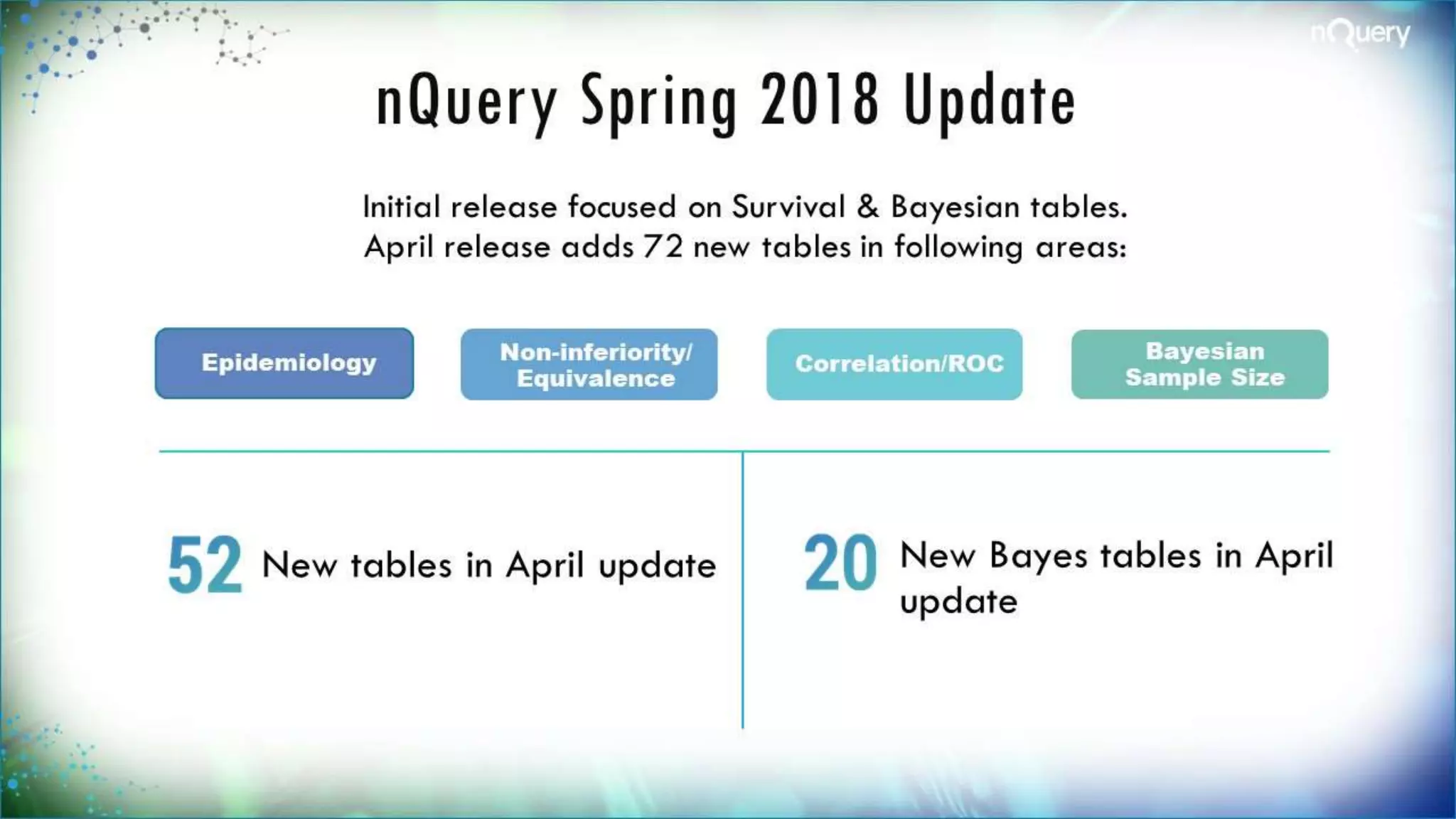
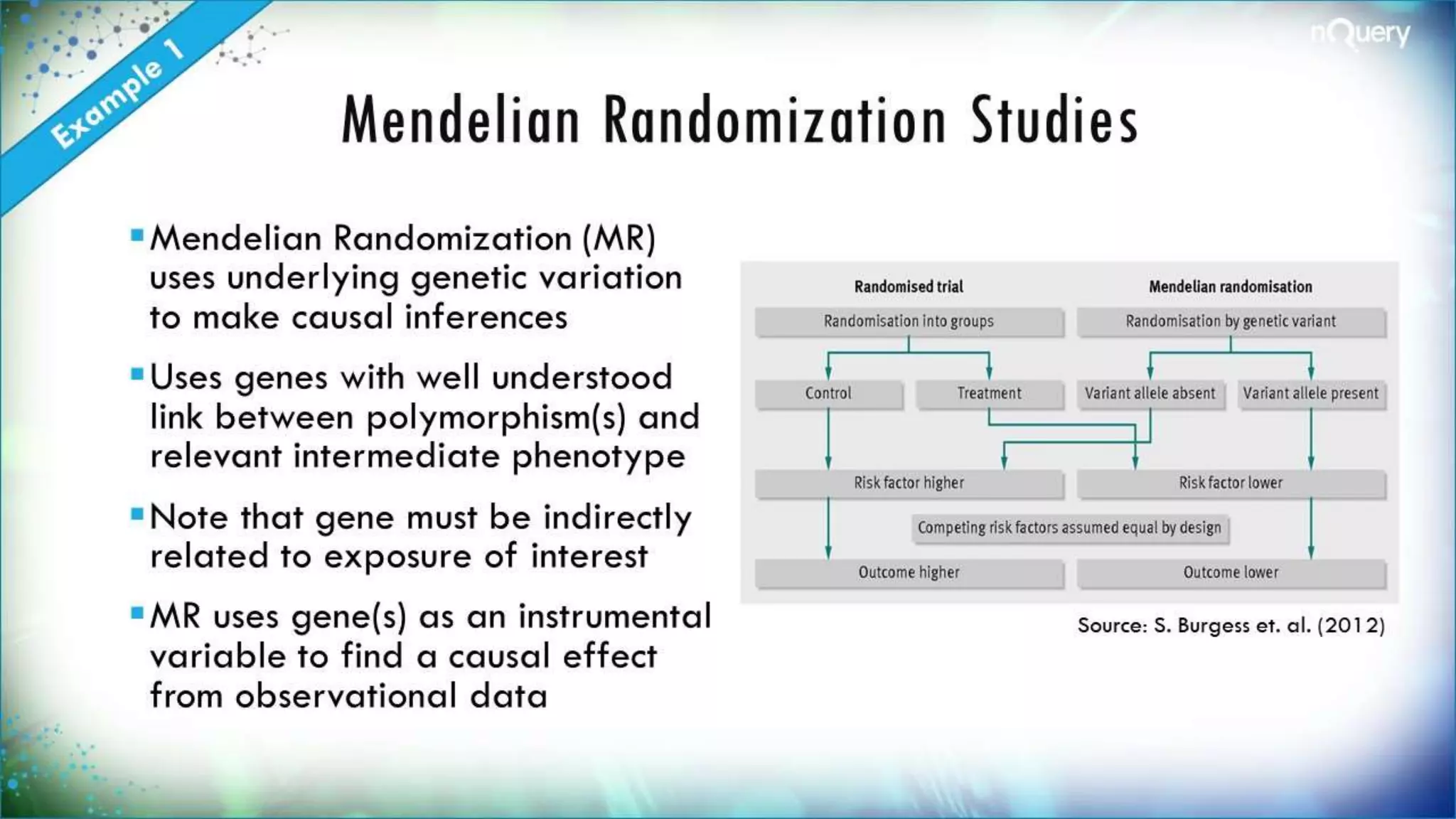
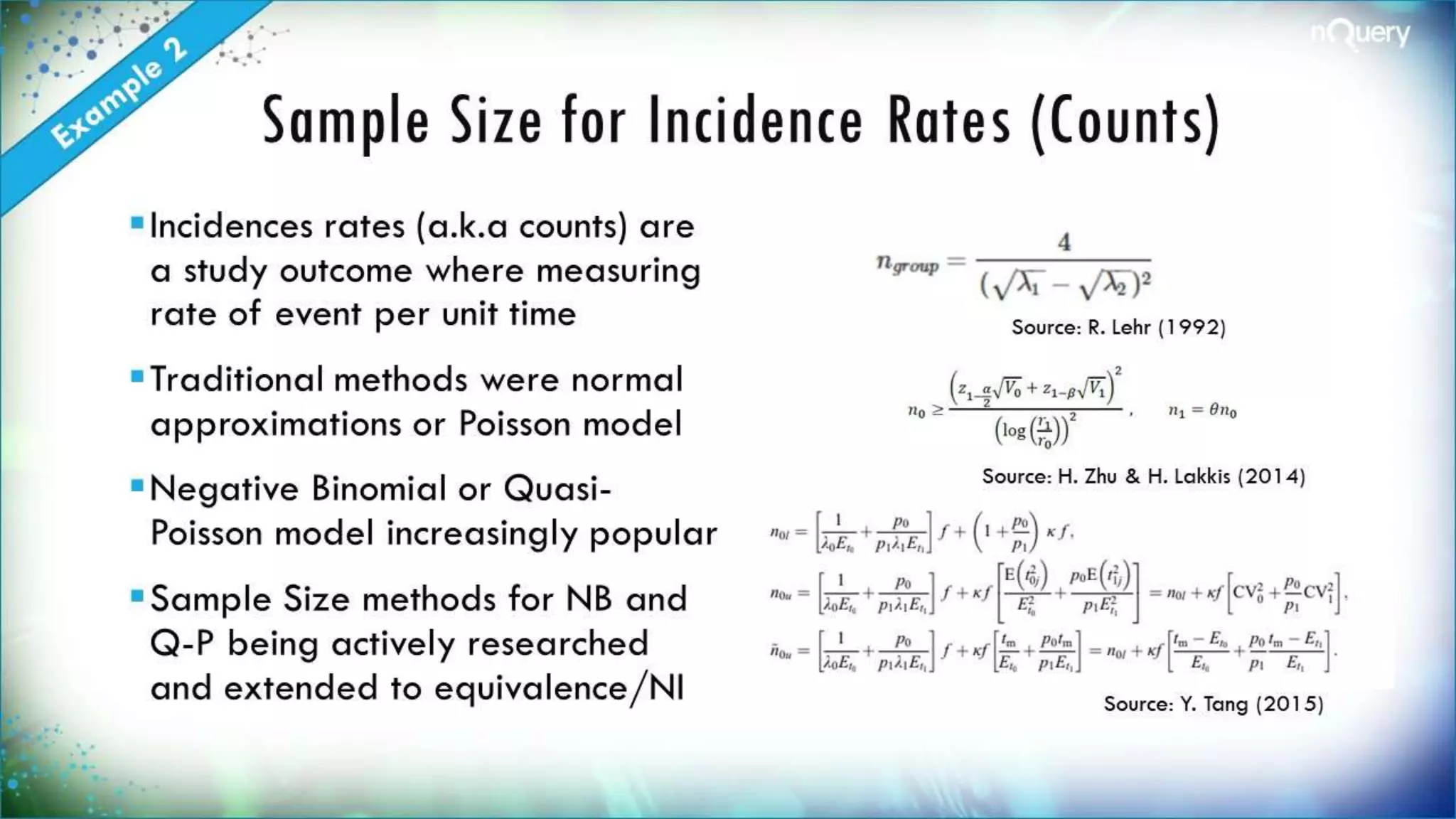
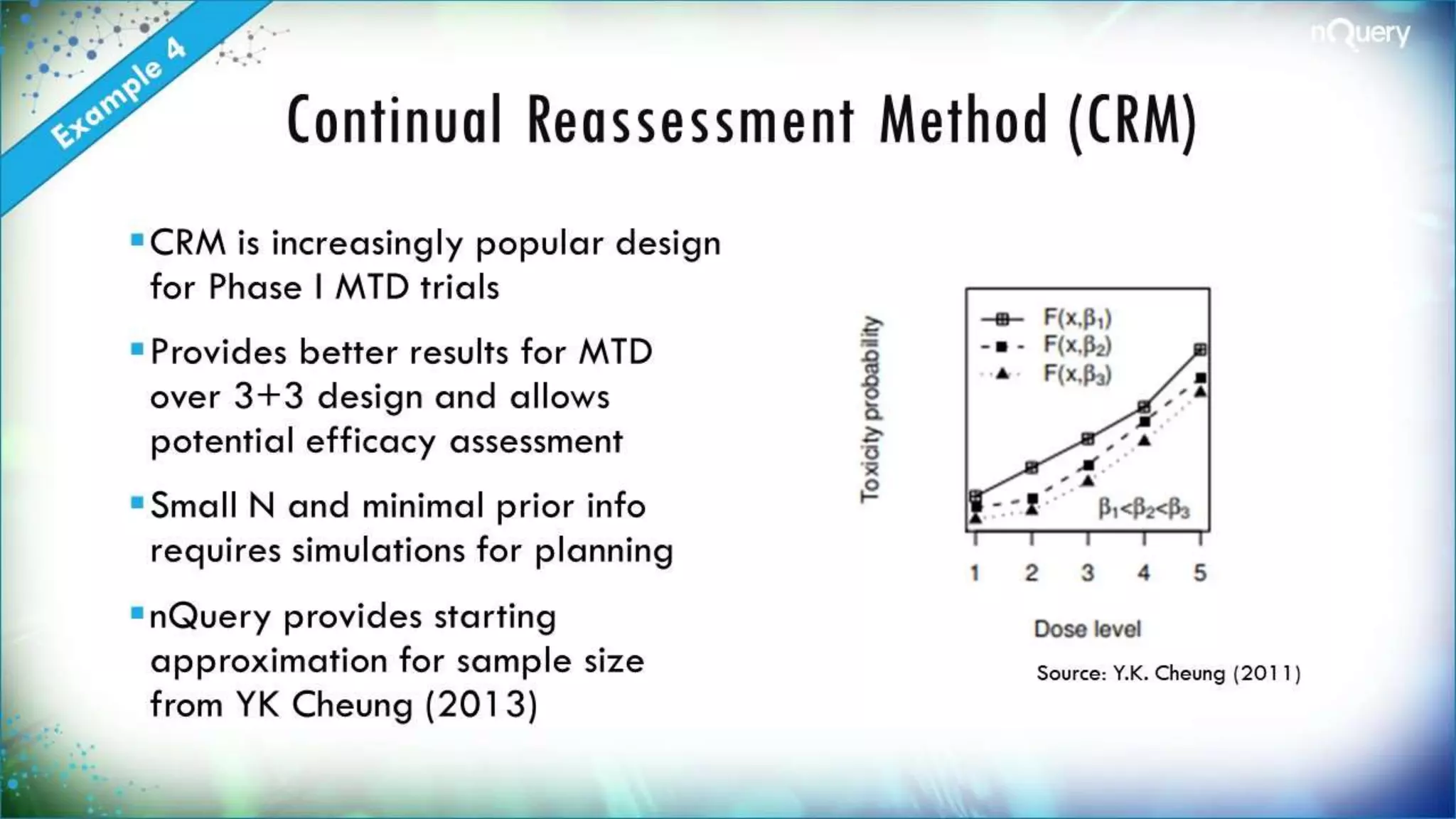
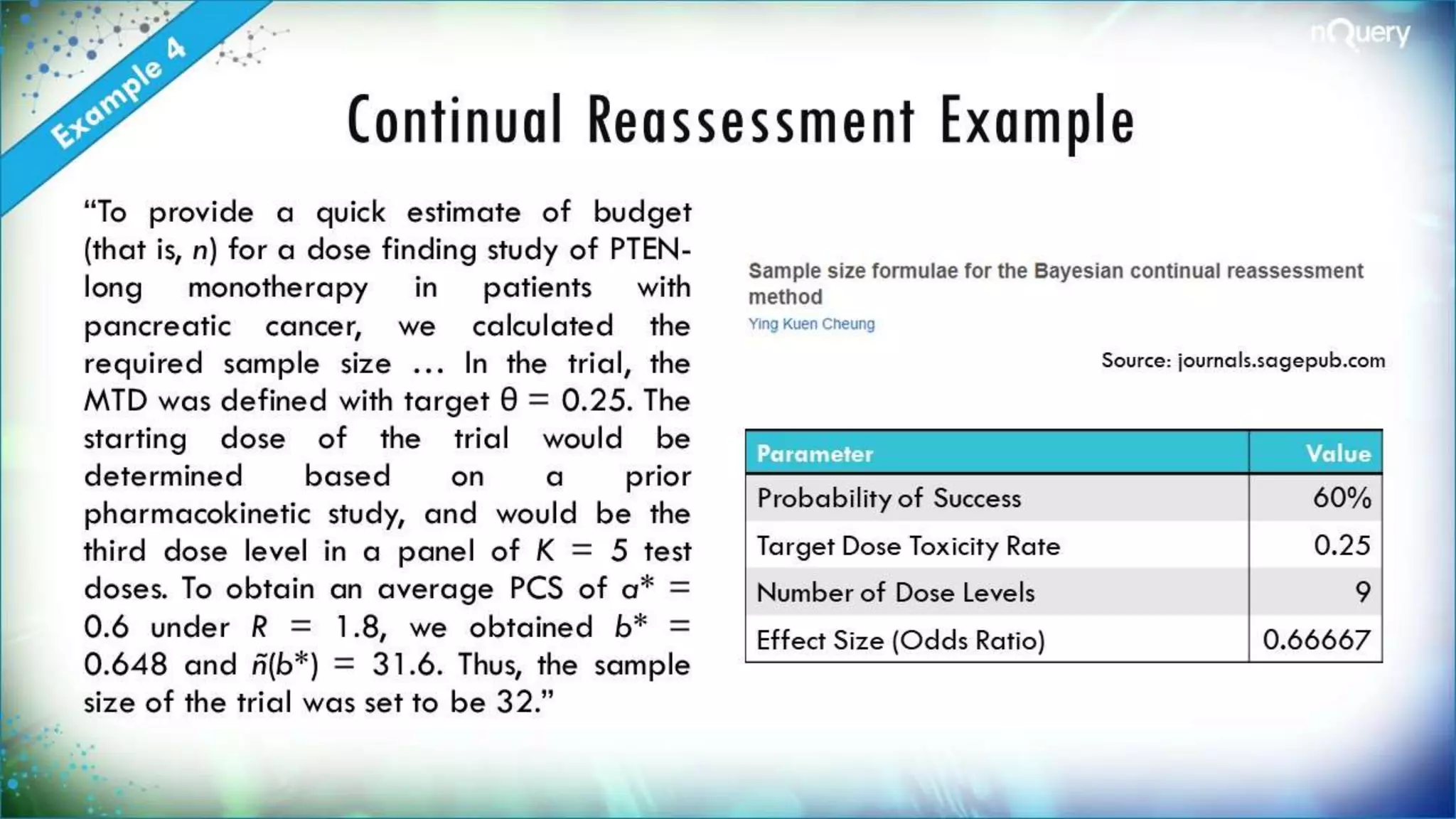
The document discusses the importance of sample size determination (SSD) in statistical studies and outlines innovative methods and challenges in SSD, particularly for clinical trials. It emphasizes various statistical techniques such as Bayesian methods and Mendelian randomization, providing examples and worked calculations to illustrate these concepts. The webinar highlights the evolution of SSD approaches, with a focus on adapting to new study designs and statistical requirements.