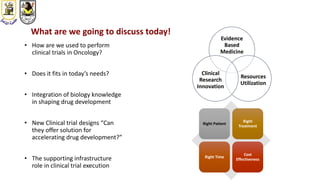
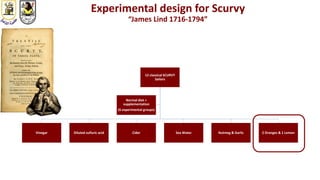
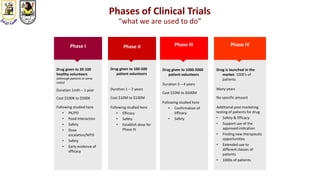
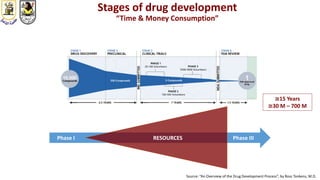
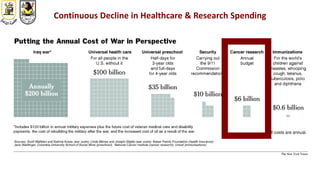
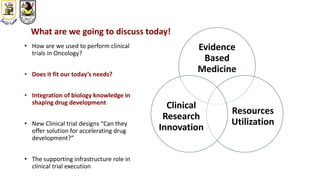
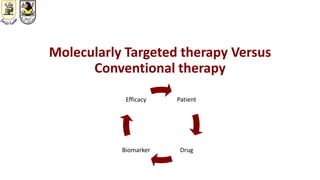
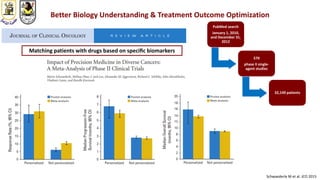
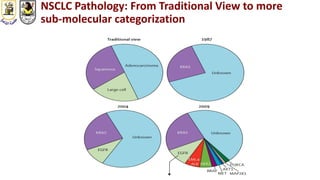
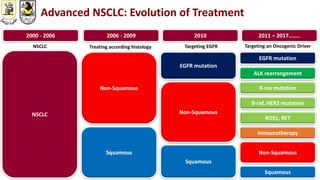
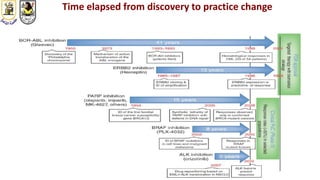
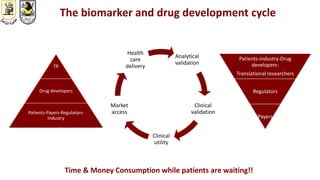
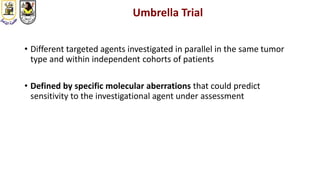
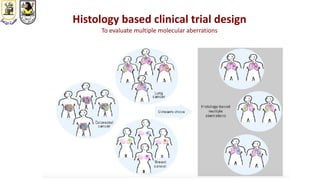
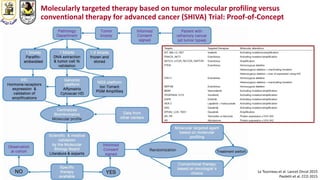
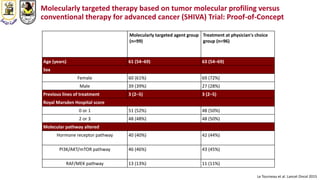
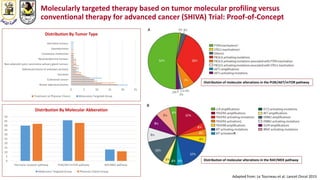
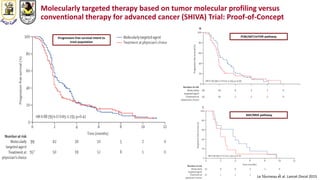
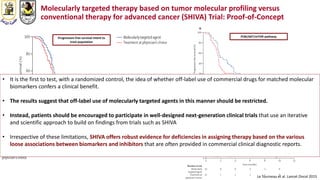
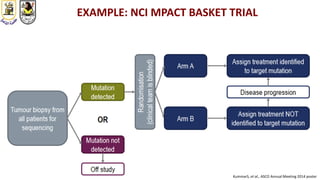
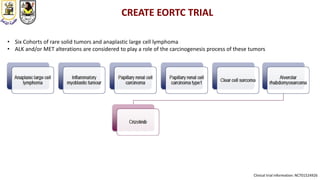
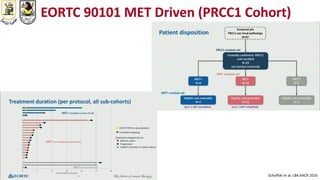
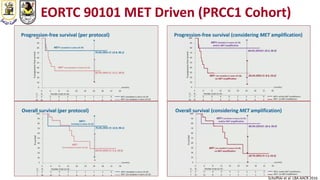
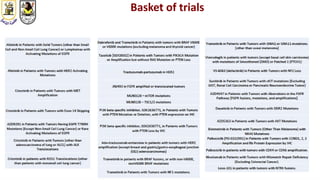
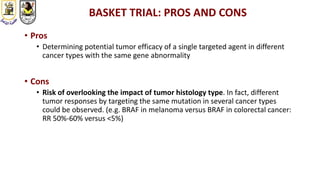
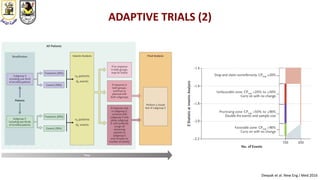
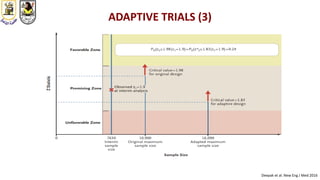
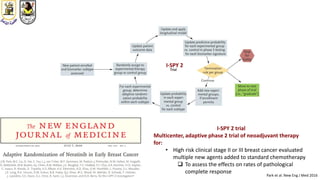
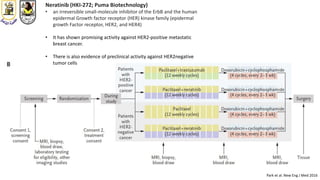
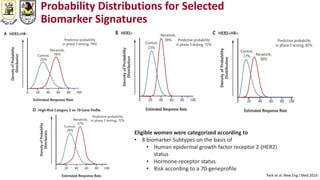
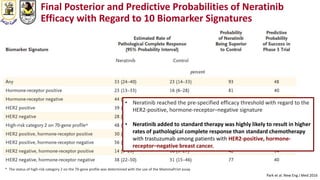
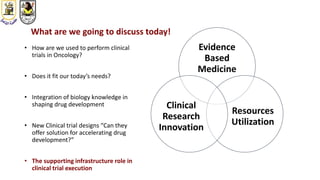
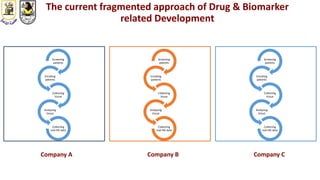
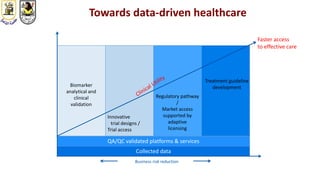
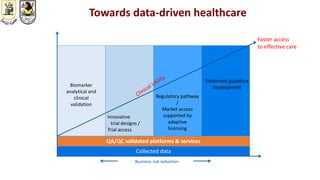
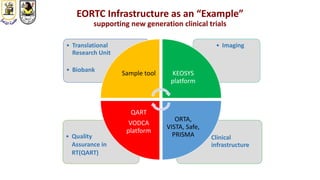
The document discusses innovative clinical trial designs aimed at maximizing benefits in oncology, particularly through evidence-based medicine and integration of biological knowledge in drug development. It evaluates traditional and new methodologies, such as adaptive trials and basket trials, including their advantages and challenges, to accelerate drug development while ensuring safety and efficacy. The conclusion emphasizes the necessity of a robust infrastructure and collaboration among various stakeholders in clinical research to meet evolving healthcare needs.