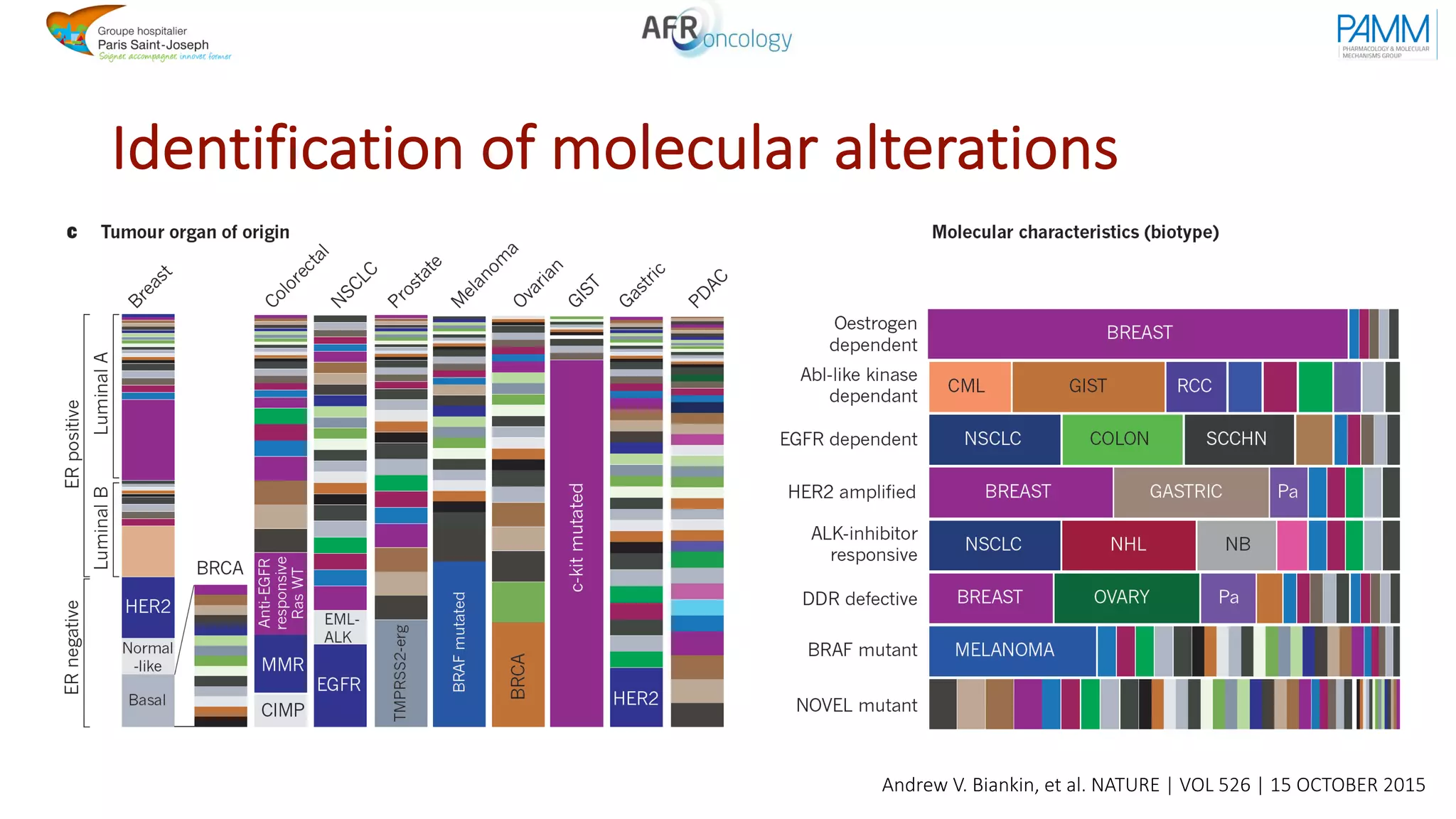
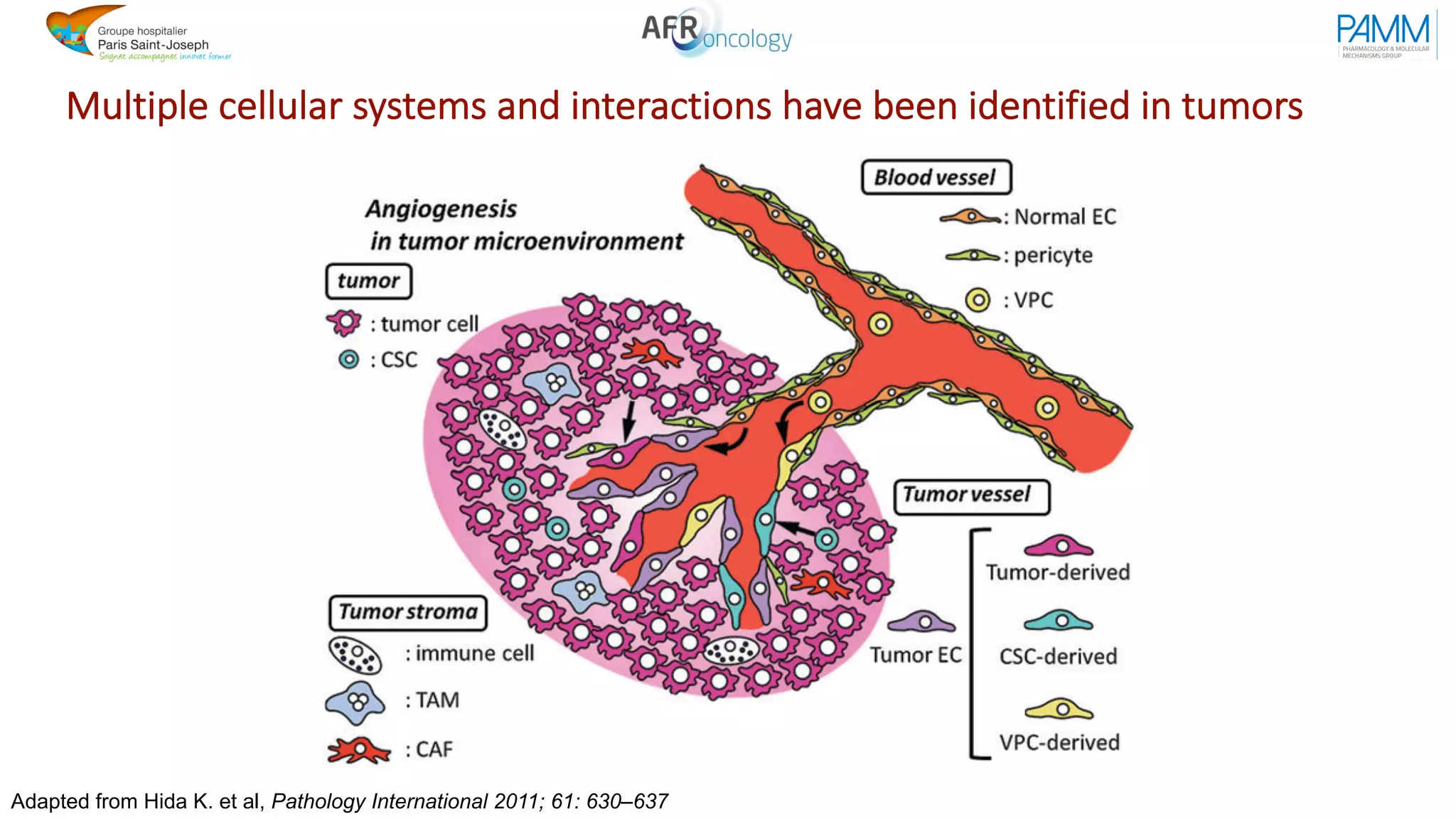
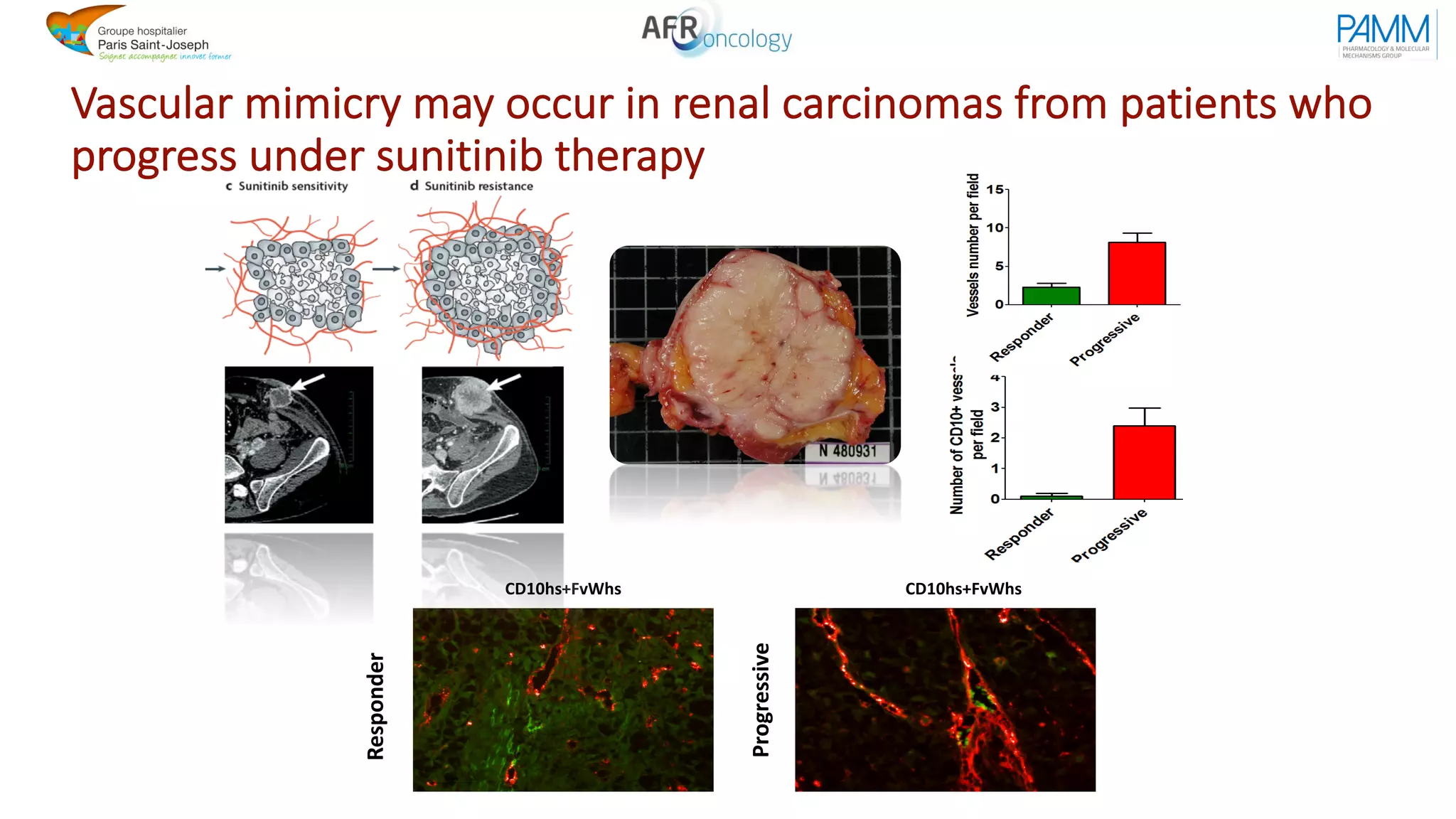
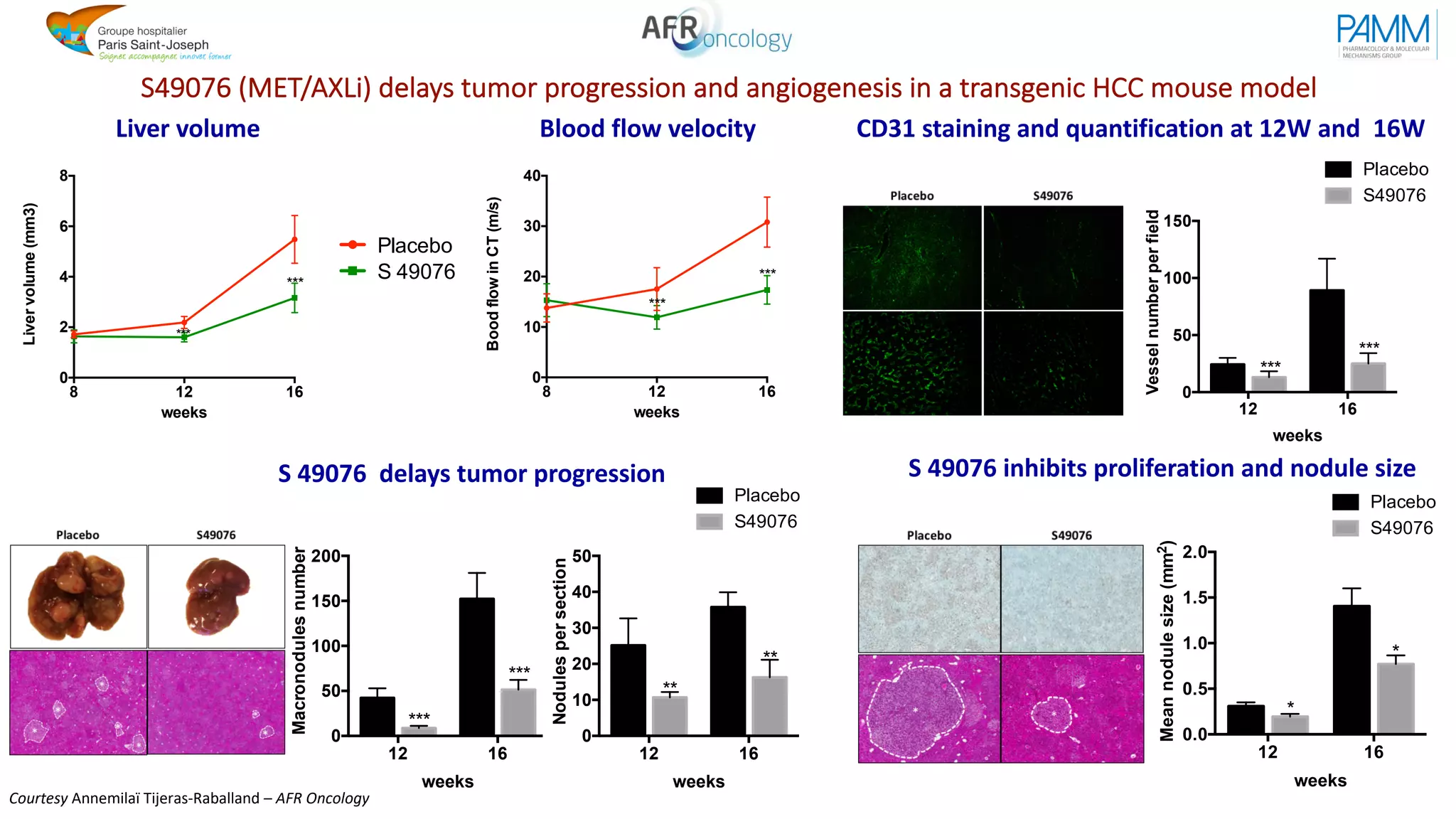
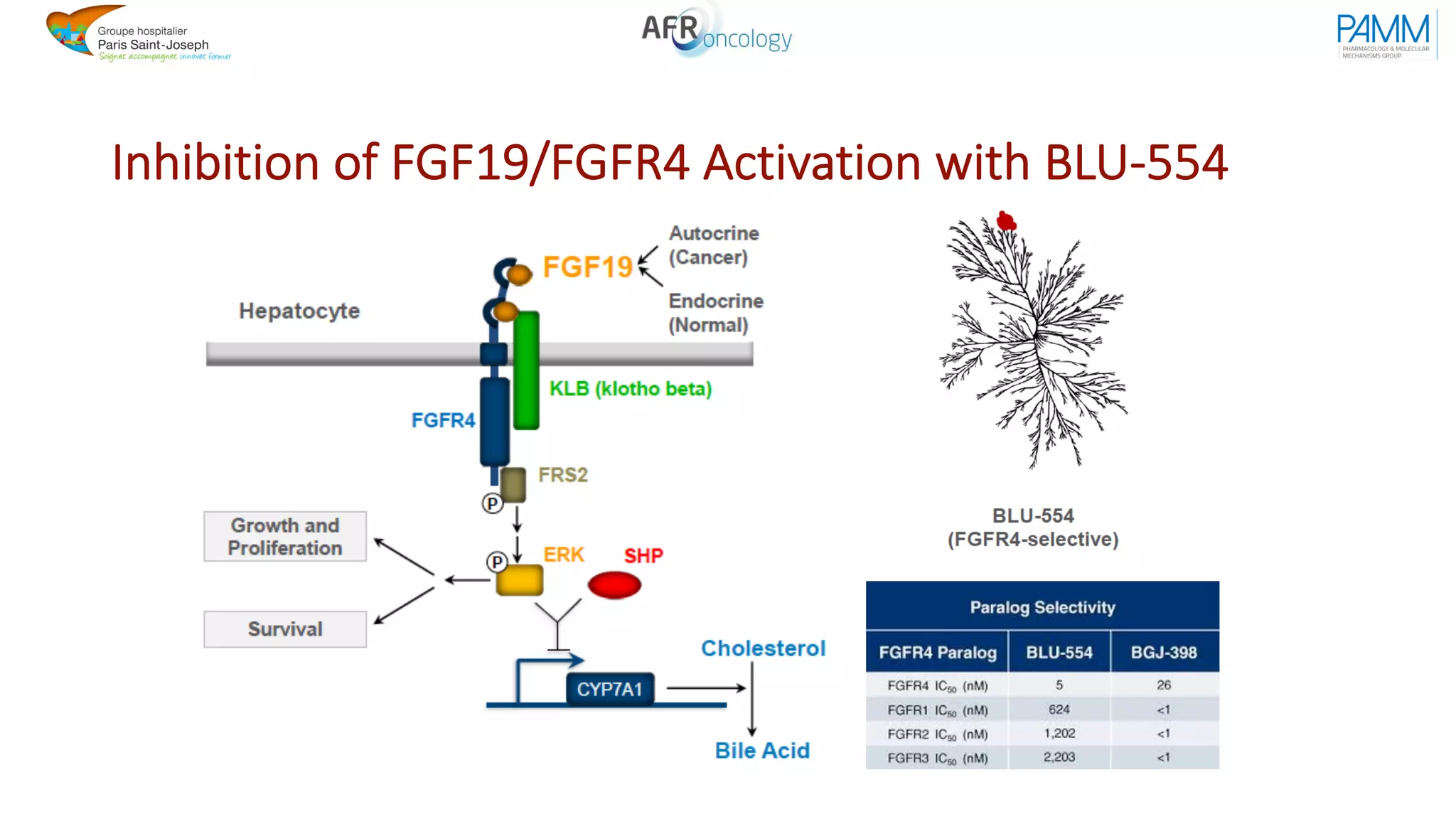
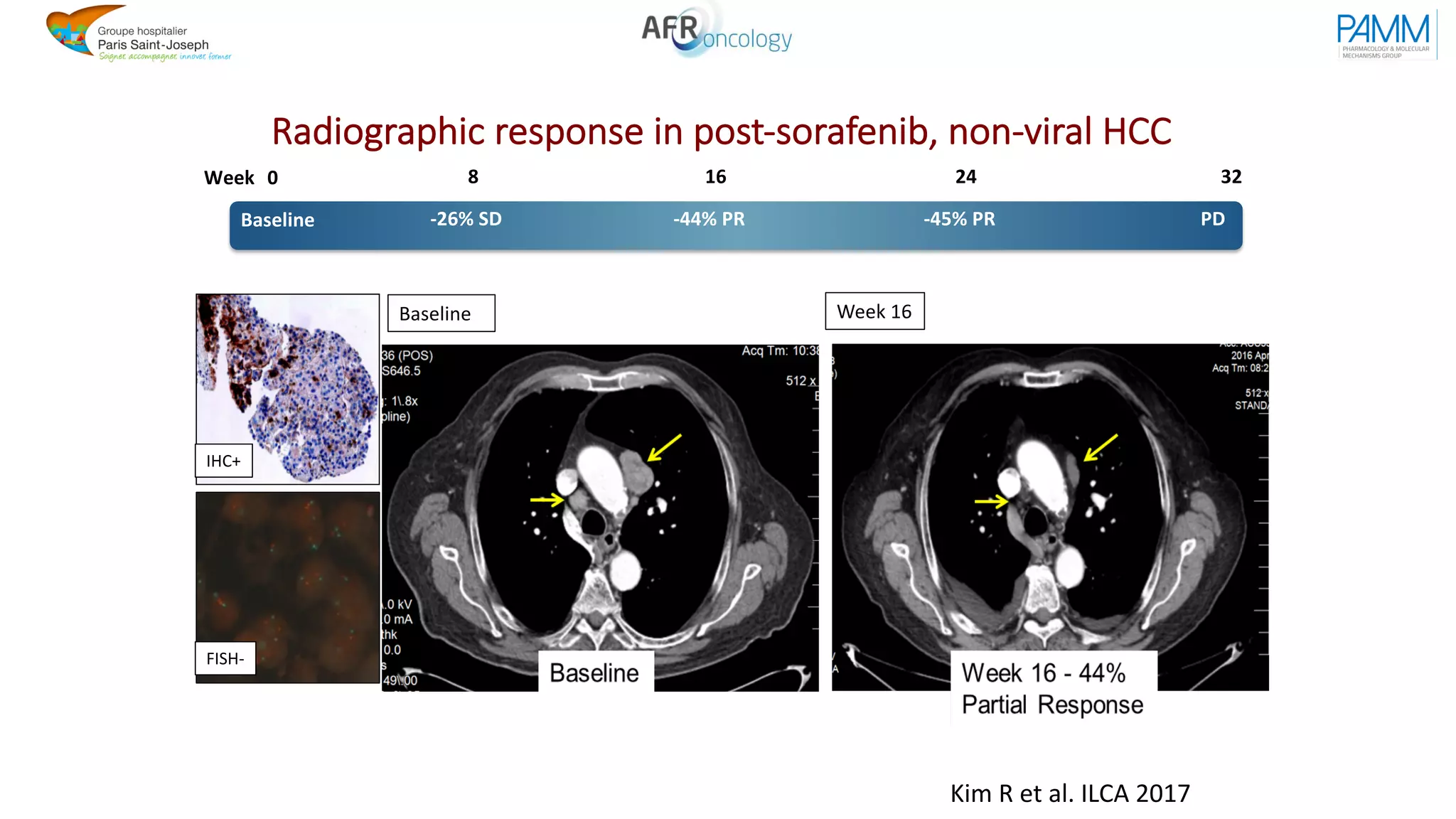
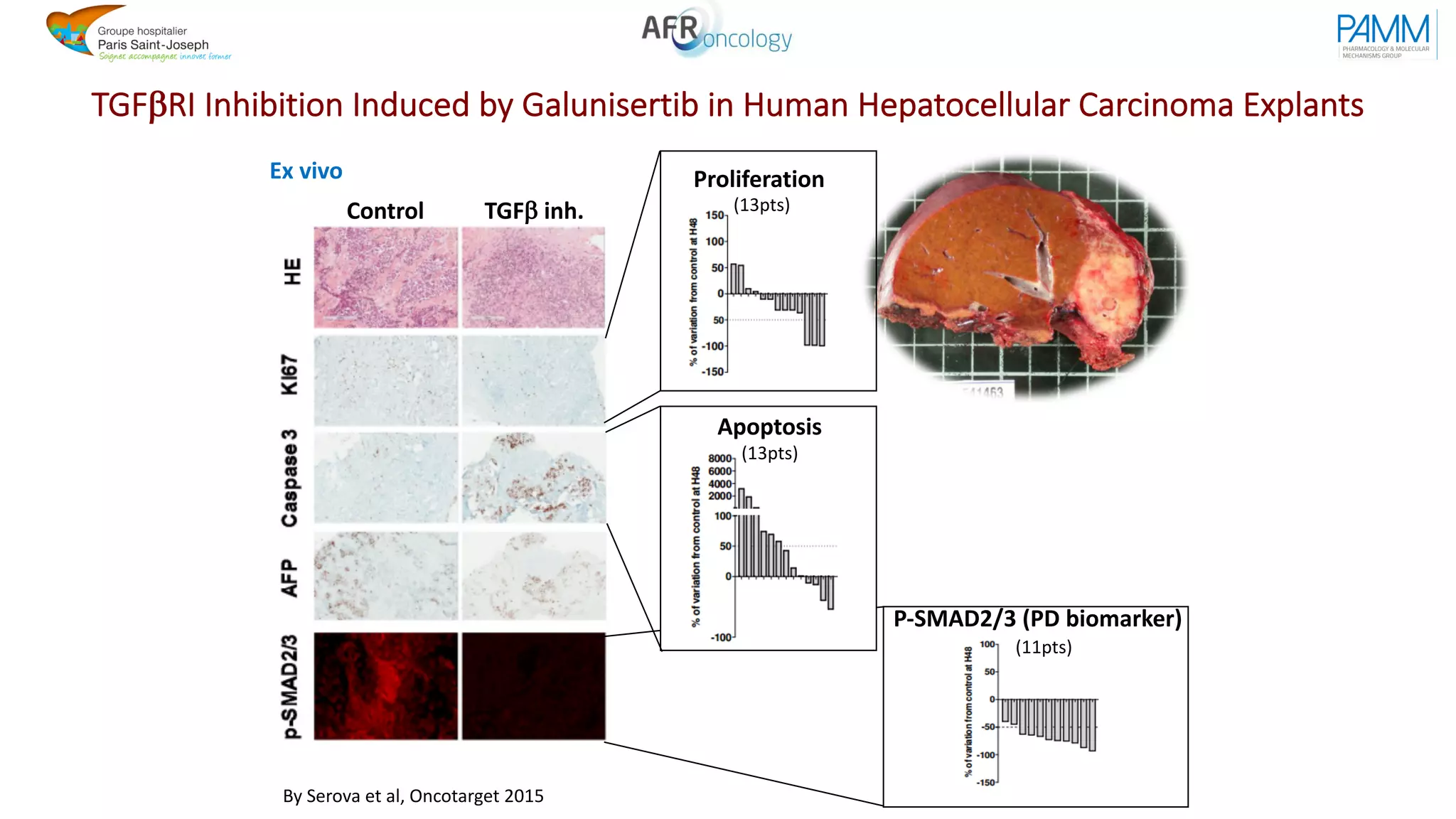
The document discusses the evolution and challenges of developing targeted cancer therapies, highlighting the roles of various agents such as kinase inhibitors and the emergence of immunotherapies. It addresses issues like tumor heterogeneity, resistance to treatments, and the need for personalized medicine through molecular profiling. Additionally, it emphasizes the importance of clinical trials in refining treatment approaches and the complexities of drug approval processes.