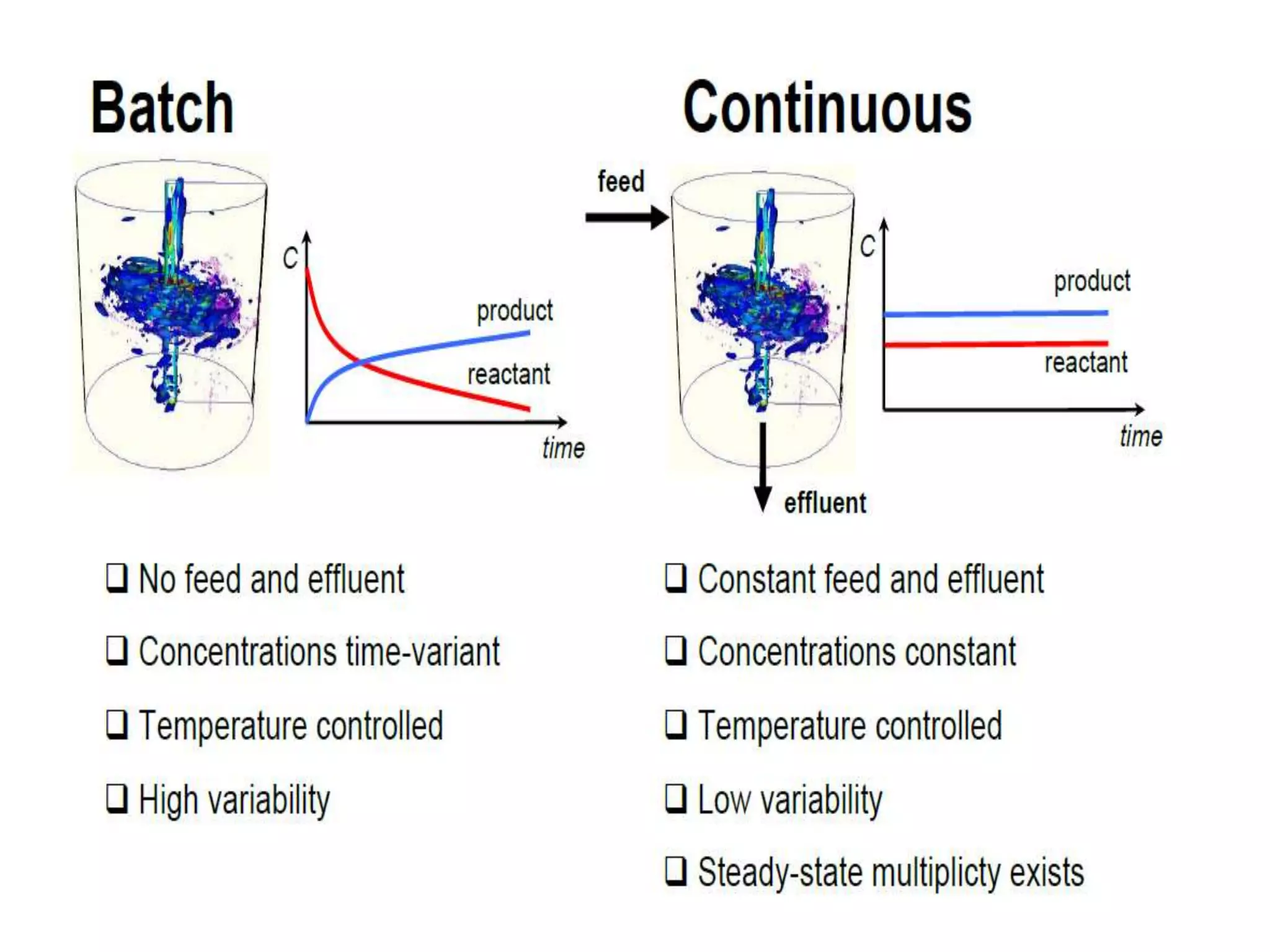
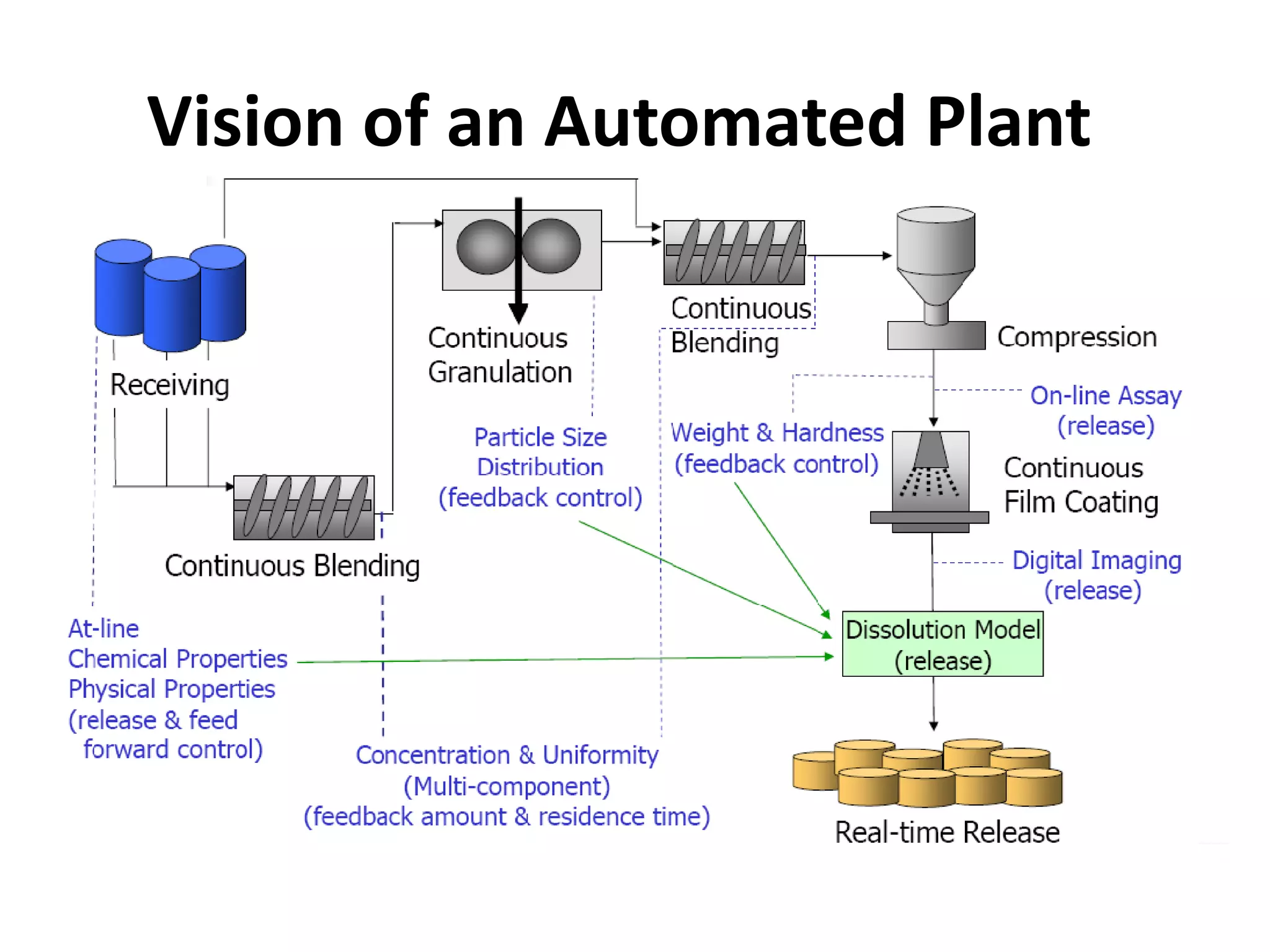
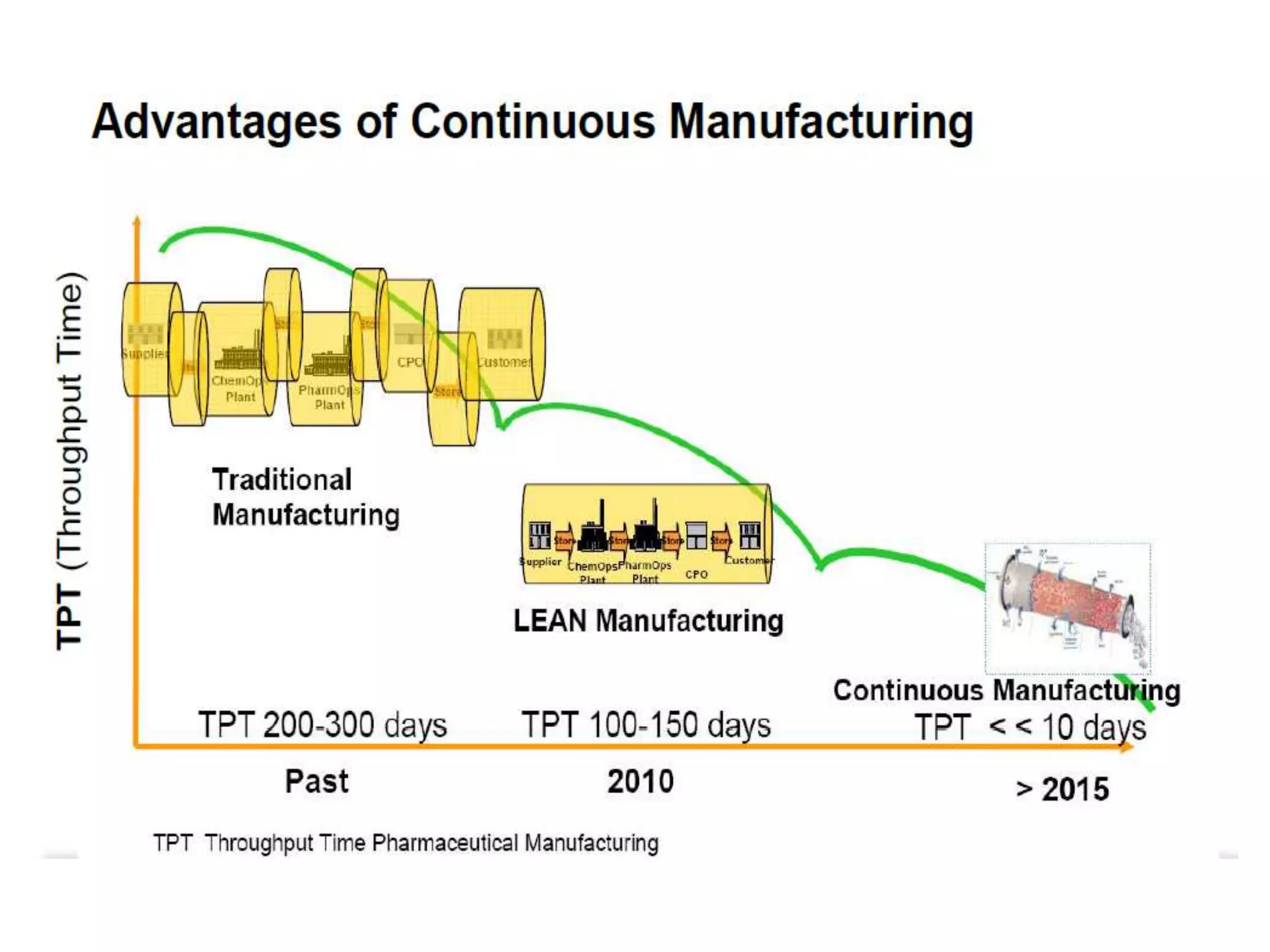
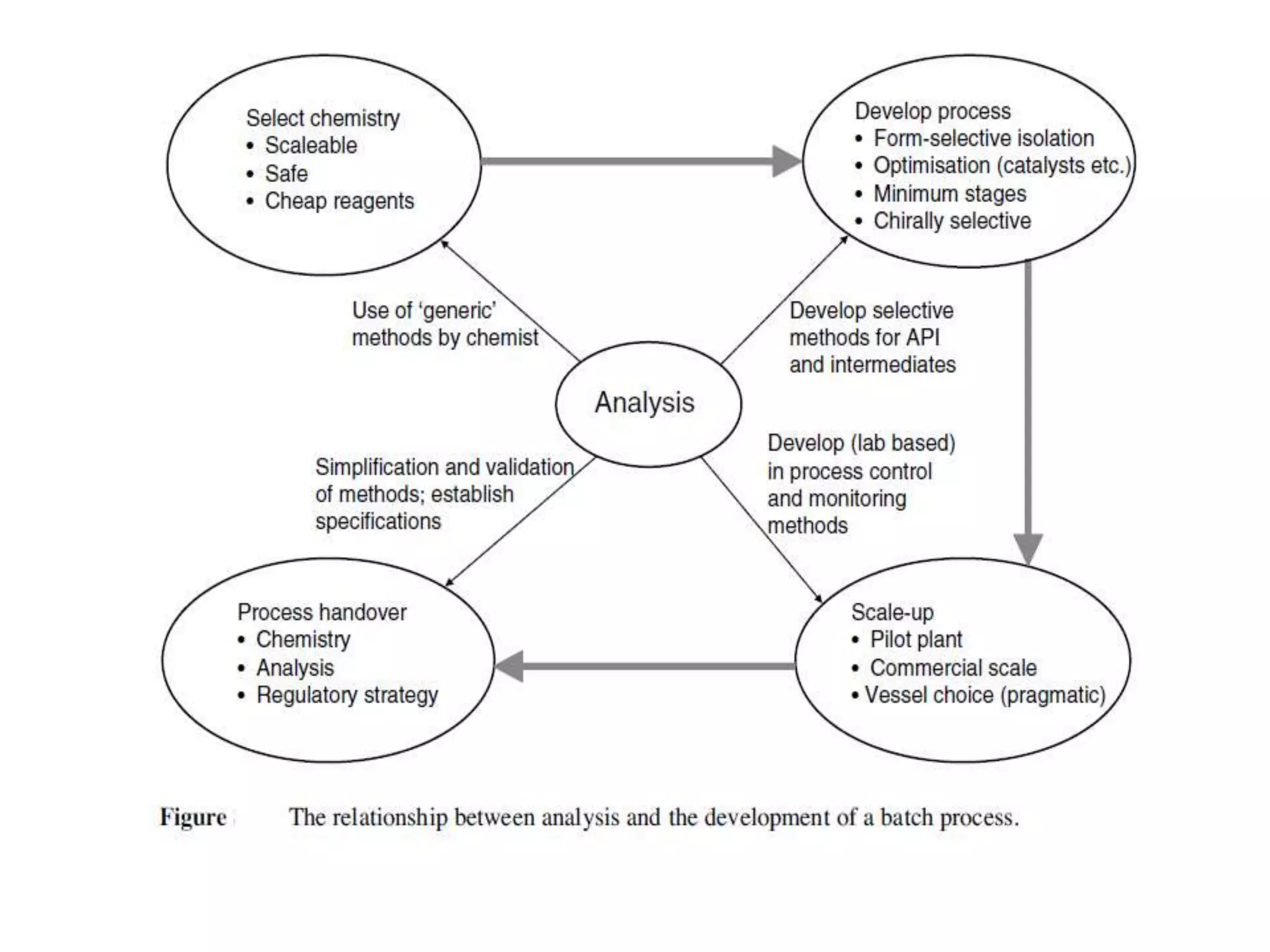
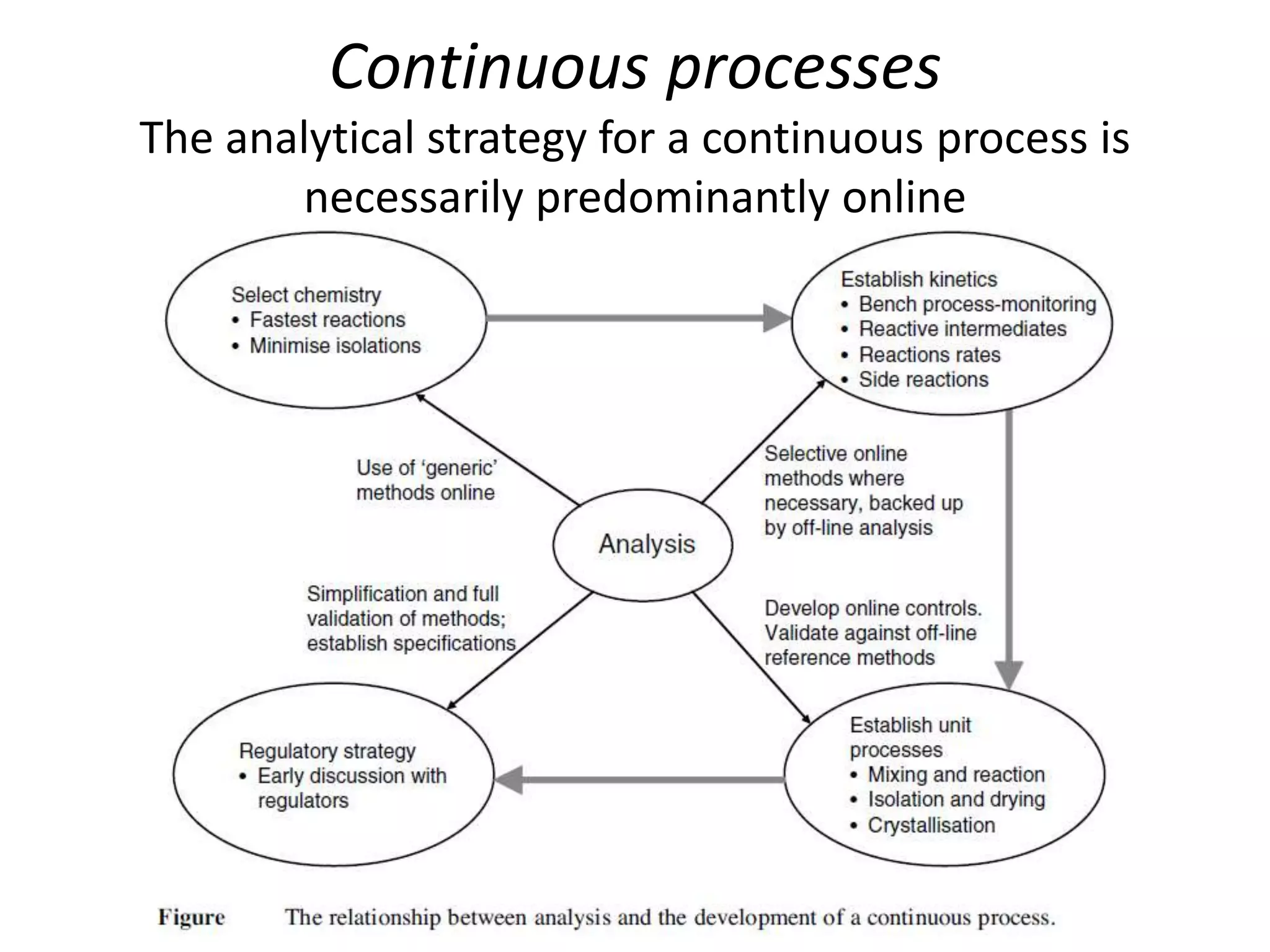
This document discusses continuous processing versus traditional batch processing in pharmaceutical manufacturing. Some key advantages of continuous processing include reduced footprint, capital and operational costs, lower raw material and intermediate inventories, and less complex scale-up. However, continuous processing also faces challenges like not being suitable for every process, requiring dedicated equipment and advanced process control approaches. The document emphasizes that process analytical technology (PAT) will play an important role in continuous manufacturing by enabling online measurement and process monitoring to ensure product quality.