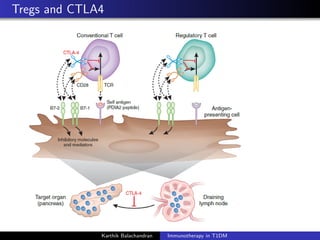
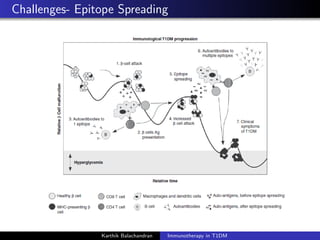
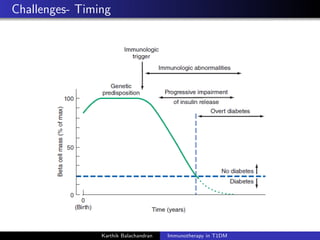
The document summarizes current immunotherapies being developed for the treatment of type 1 diabetes. It describes how autoimmunity leads to the destruction of beta cells and the goals of immunotherapy to slow or stop this process. Several antigen-specific therapies targeting proteins like GAD, insulin, and HSP60 as well as monoclonal antibodies, fusion proteins, and Treg affectors are discussed. Clinical trials for many of these candidates showed only transient effects in preserving beta cell function and glycemic control with no therapy providing a lasting halt to the autoimmune attack. Combination therapies and identifying the best patient populations may improve outcomes but ideal timing and dosing remain unclear.