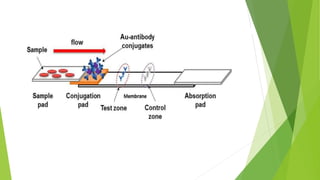
Immunoassays are chemical tests that use antibodies and antigens to detect or quantify specific substances in body fluids. They are highly sensitive and specific. Immunoassays can be qualitative, detecting only presence or absence, or quantitative, measuring exact concentrations. They are used in forensic toxicology to screen samples for drugs and toxins. The introduction of immunoassays in the 1970s greatly increased the speed and efficiency of toxicology screening. While most are developed for urine, forensic toxicologists apply immunoassays to other matrices like blood and tissue. Interpretation requires considering detection limits, cross-reactivity with other substances, and potential interference from matrices or added compounds. Immunoassays provide a rapid screening tool, but confirmation with