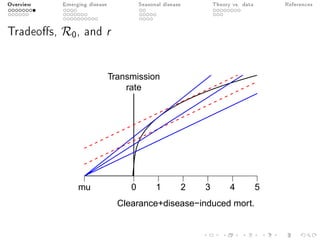
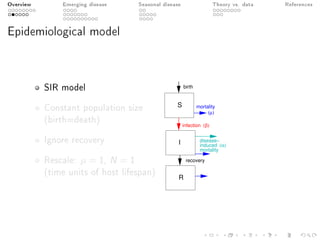
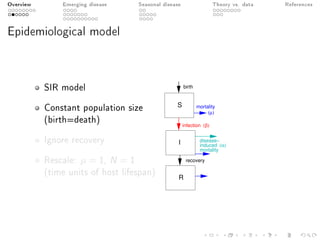
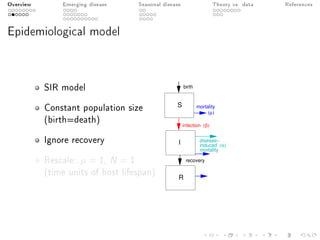
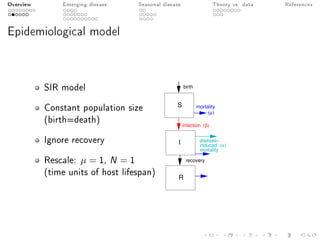
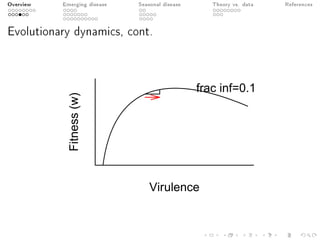
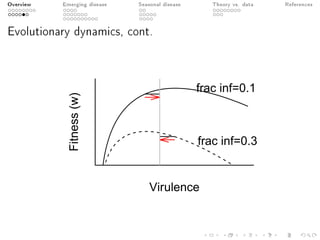
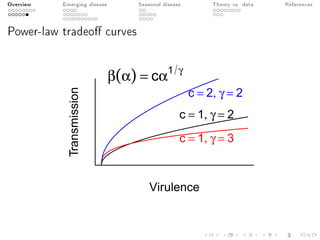
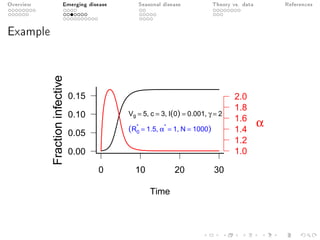
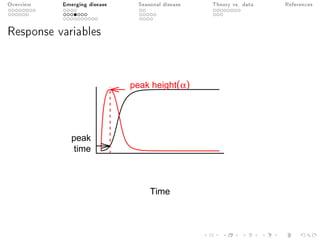
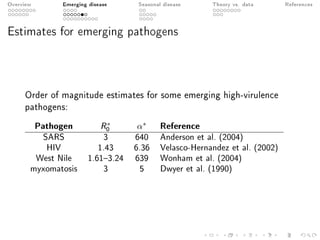
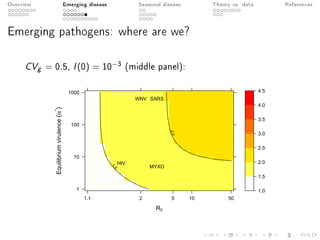
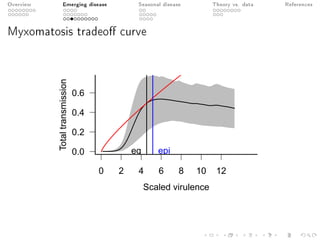
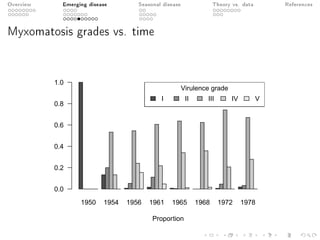
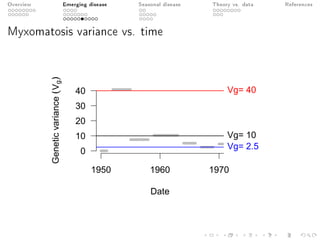
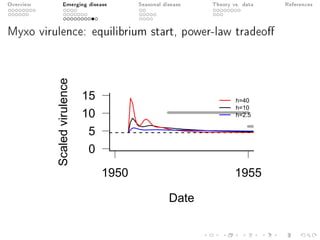
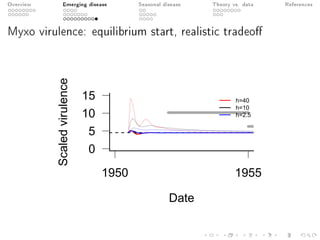
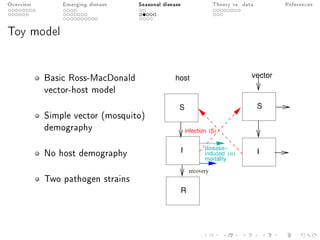
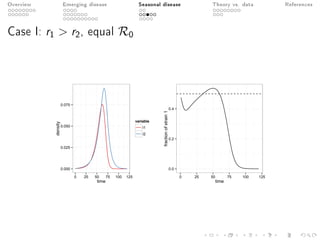
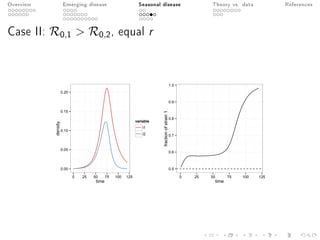
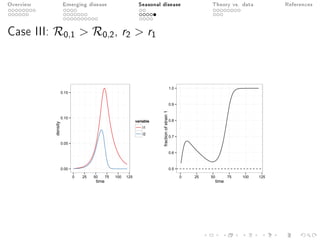
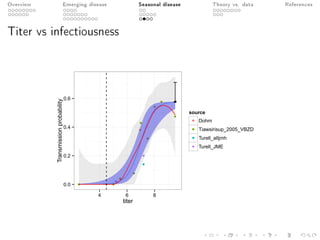
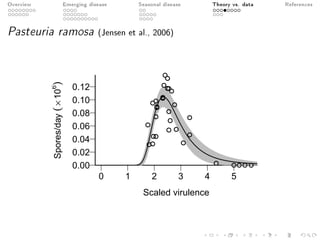
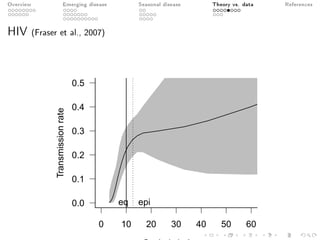
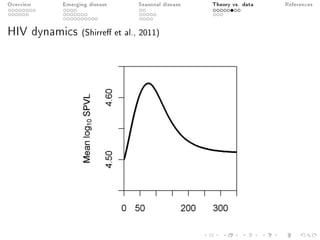
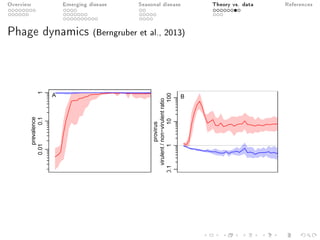
This document provides an overview of a presentation on eco-evolutionary models of pathogen virulence. It begins with background on the evolution of theories of host-pathogen interactions. It then presents a toy model combining epidemiological and evolutionary dynamics to examine how virulence may transiently peak when a pathogen emerges in a new host population before declining. The model is used to explore how the initial epidemic size and genetic variation affect the height and timing of the virulence peak. The discussion considers how the model may help explain observed patterns of high initial virulence sometimes seen in emerging infectious diseases.