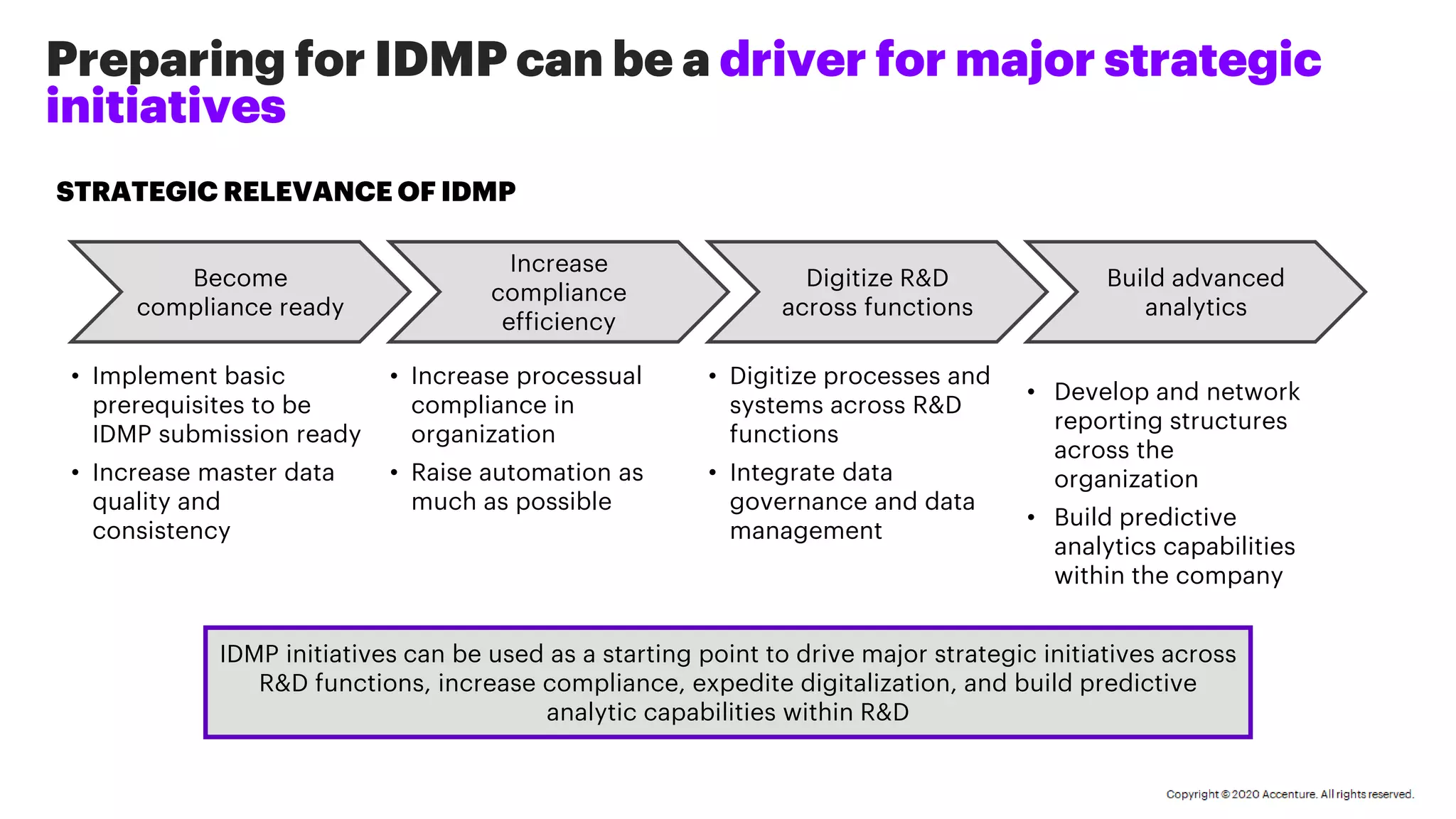
This document discusses Accenture's approach to helping companies comply with IDMP (Identification of Medicinal Products) regulations through data management and technology solutions. The four-phased approach involves:
1) Analyzing and mining existing data
2) Mapping and extracting data
3) Cleansing and enriching the data
4) Collecting and maintaining IDMP-compliant data for submission
Accenture aims to increase compliance efficiency and help clients digitize processes through this data-driven approach and the use of automation and AI tools.