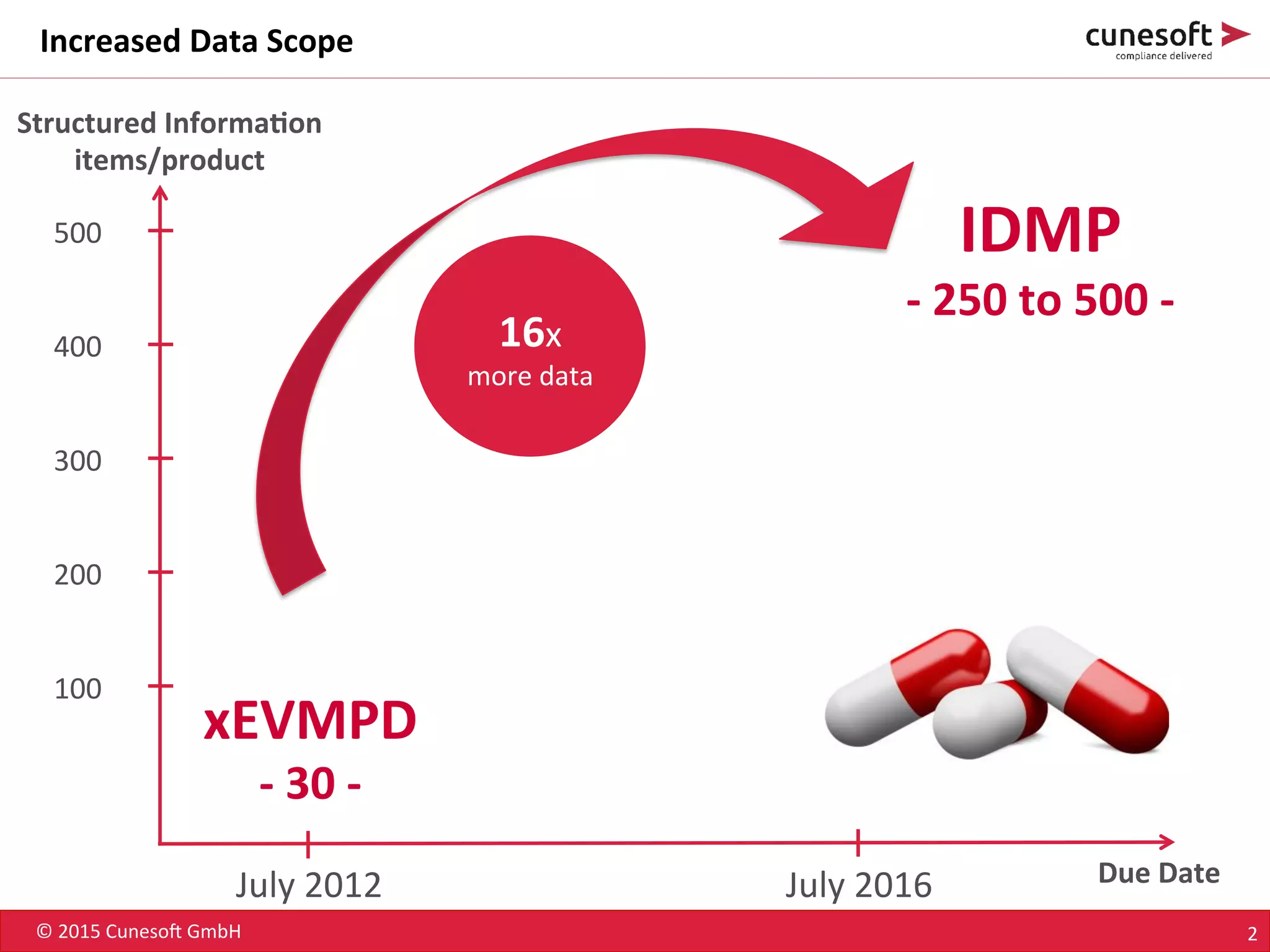
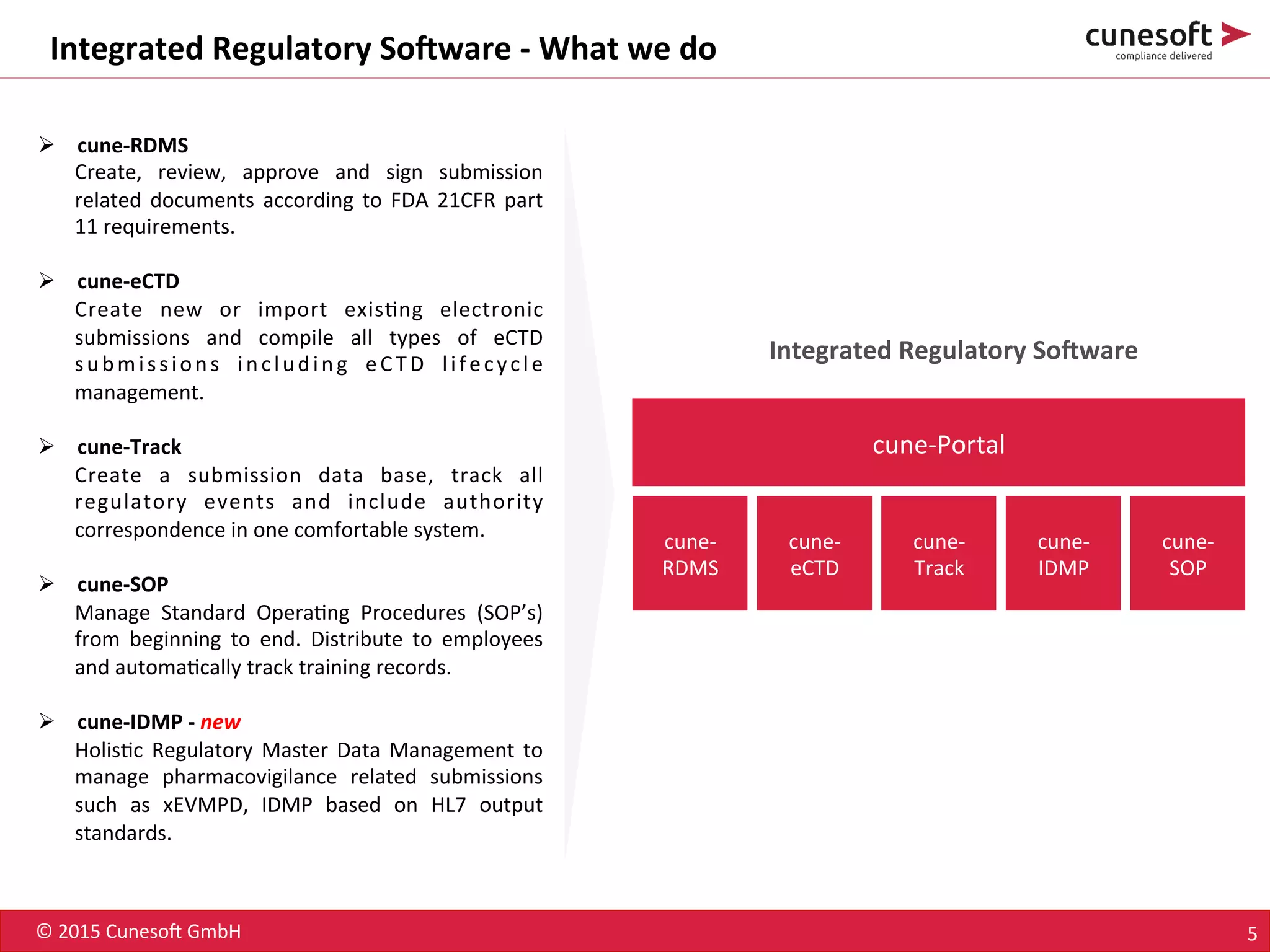
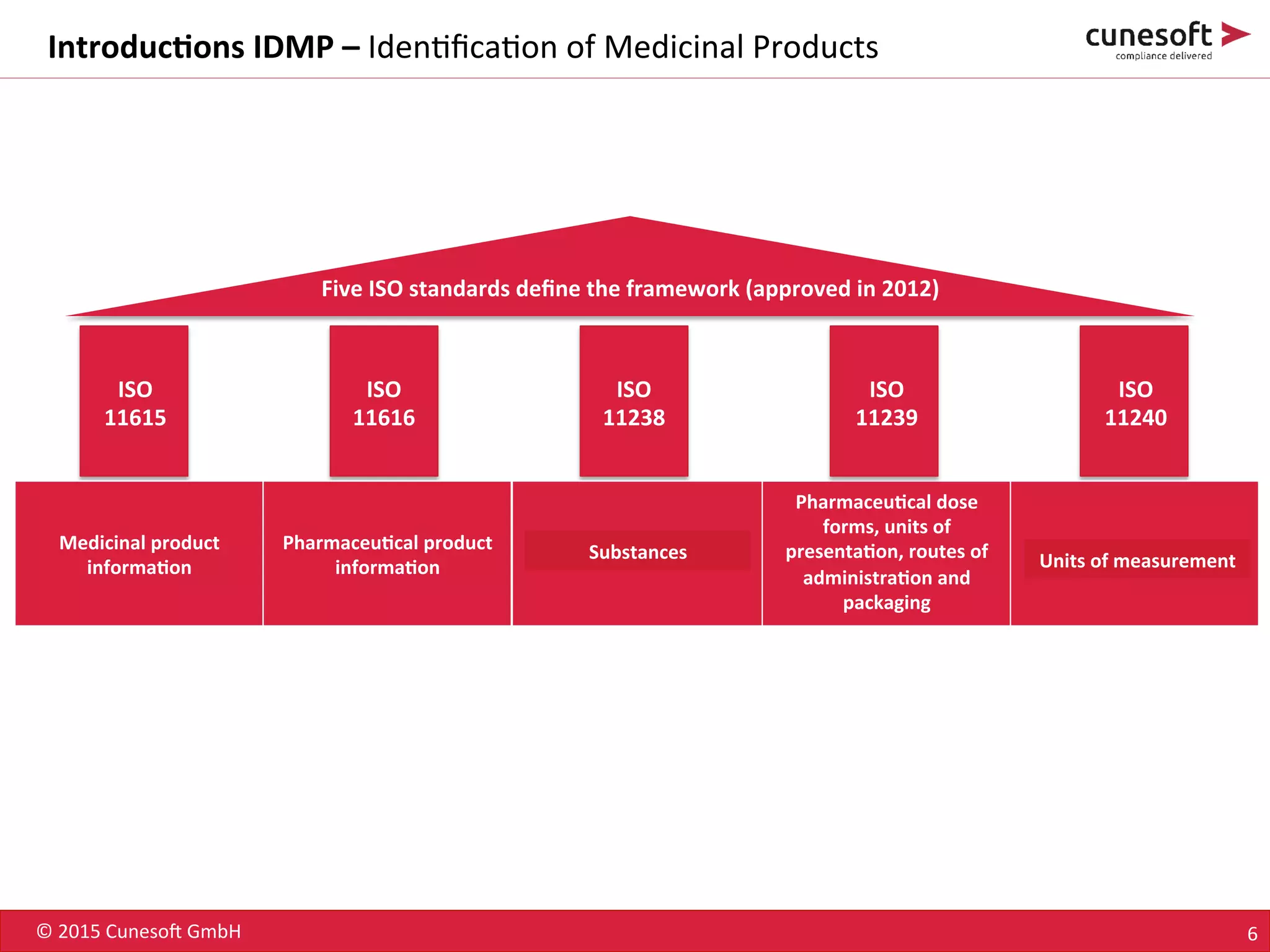
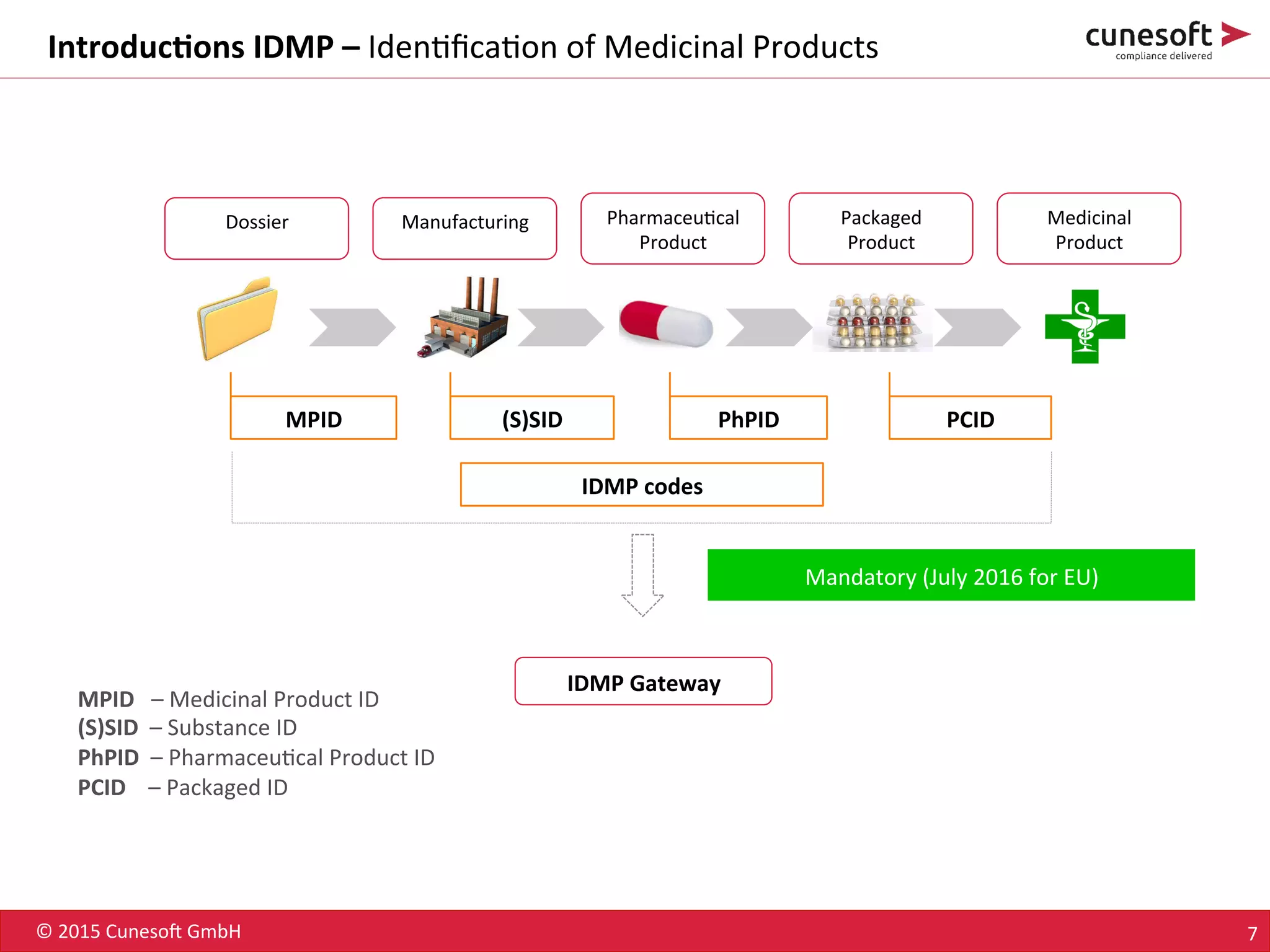
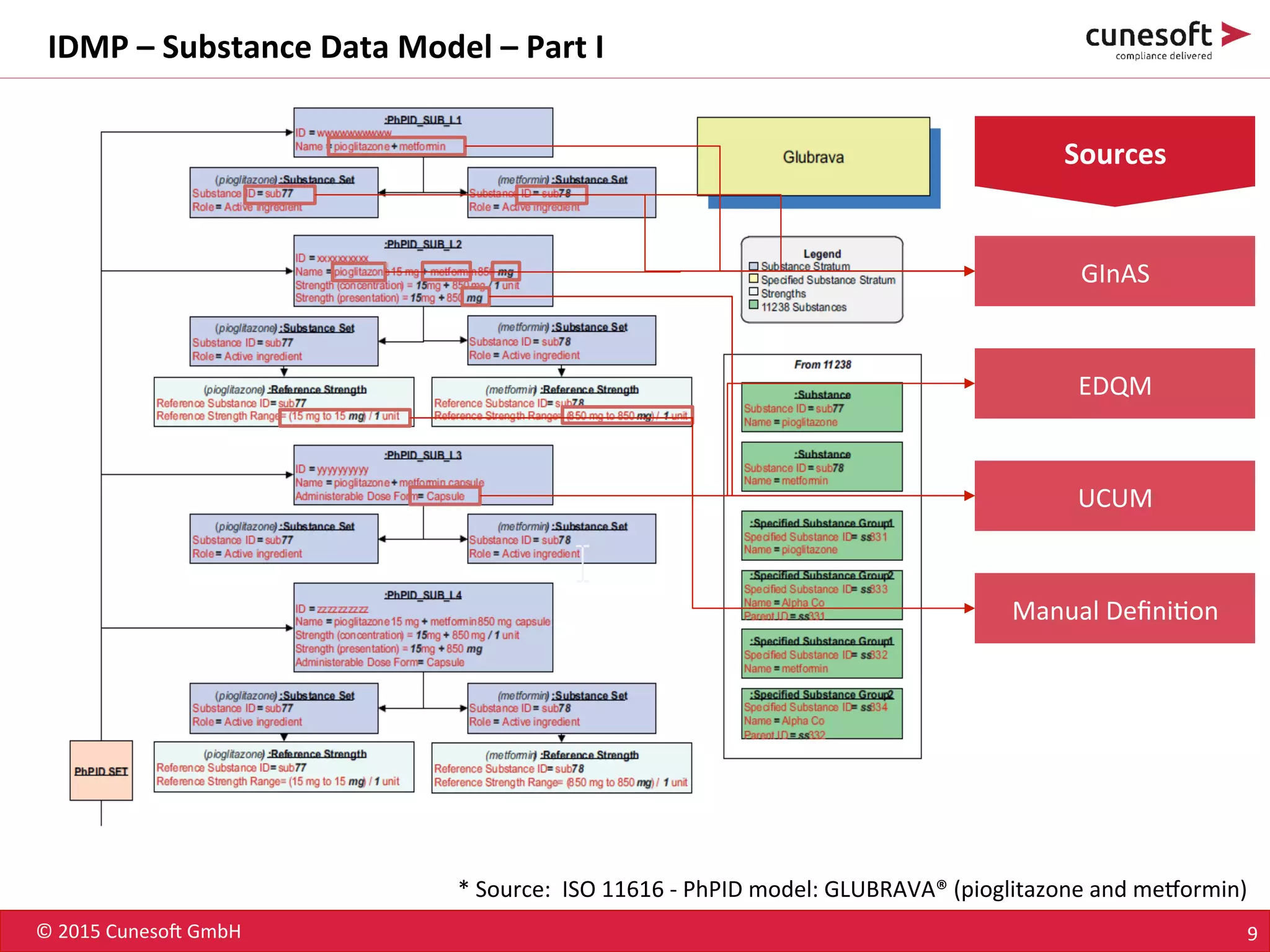
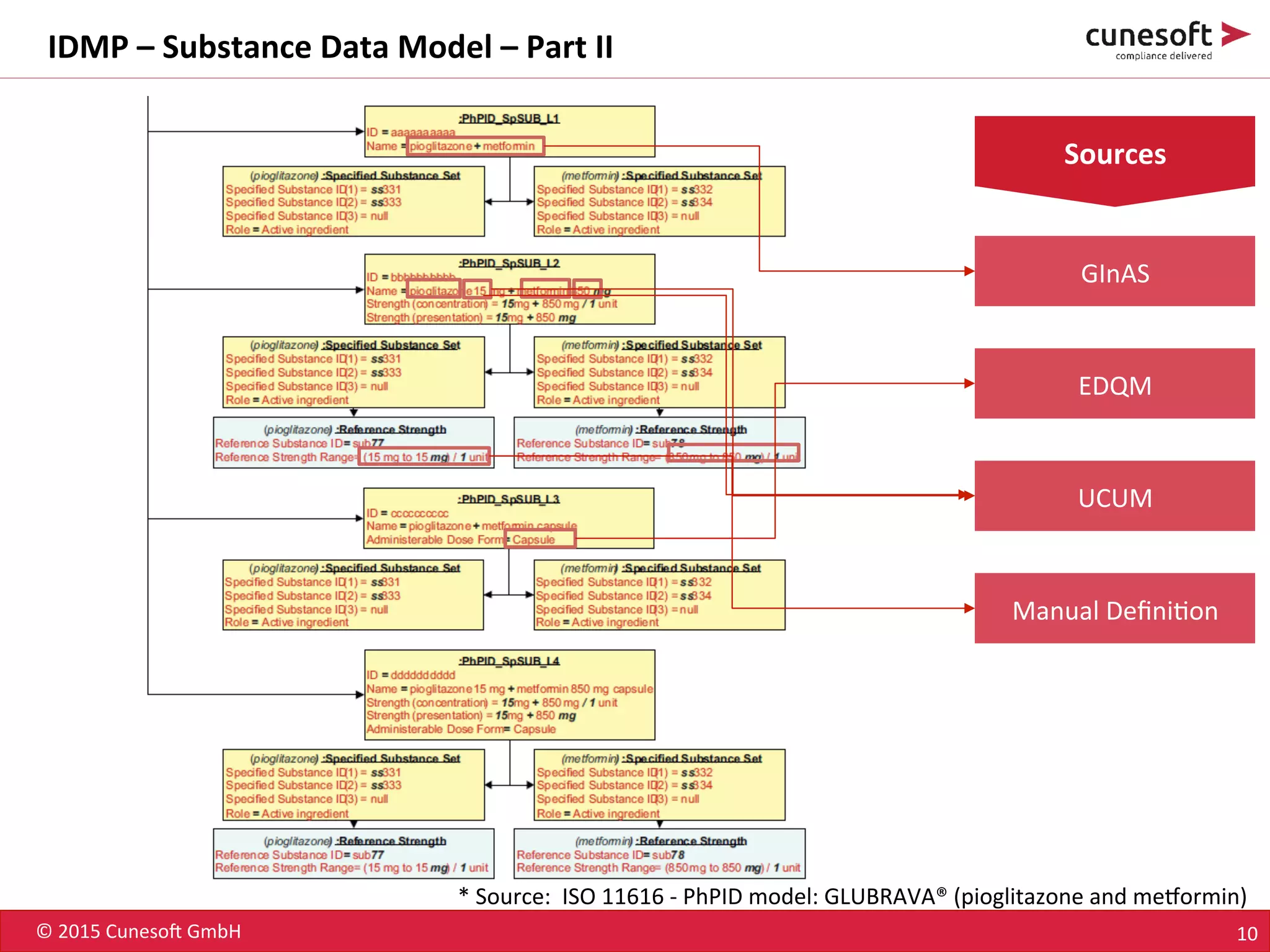
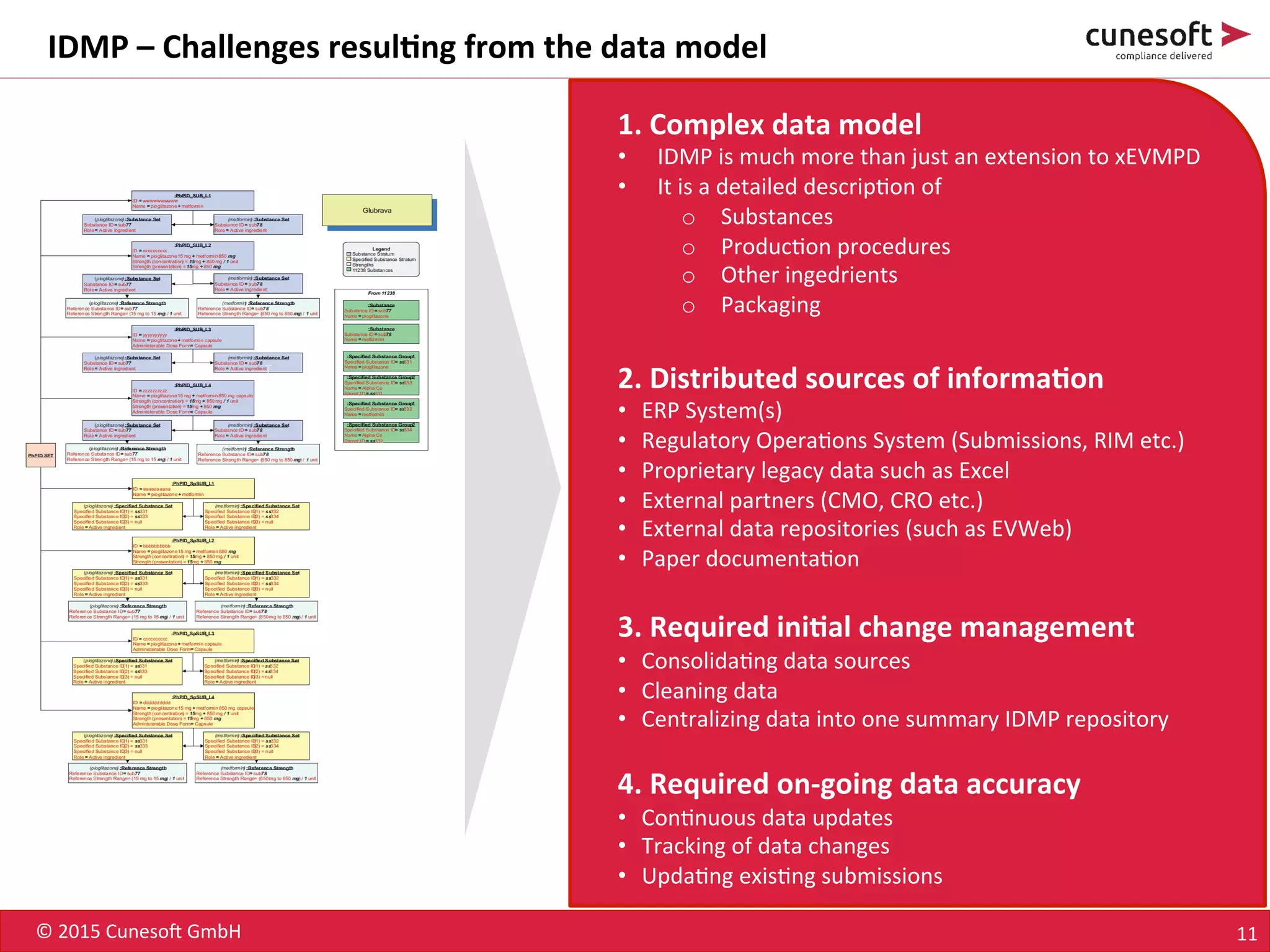
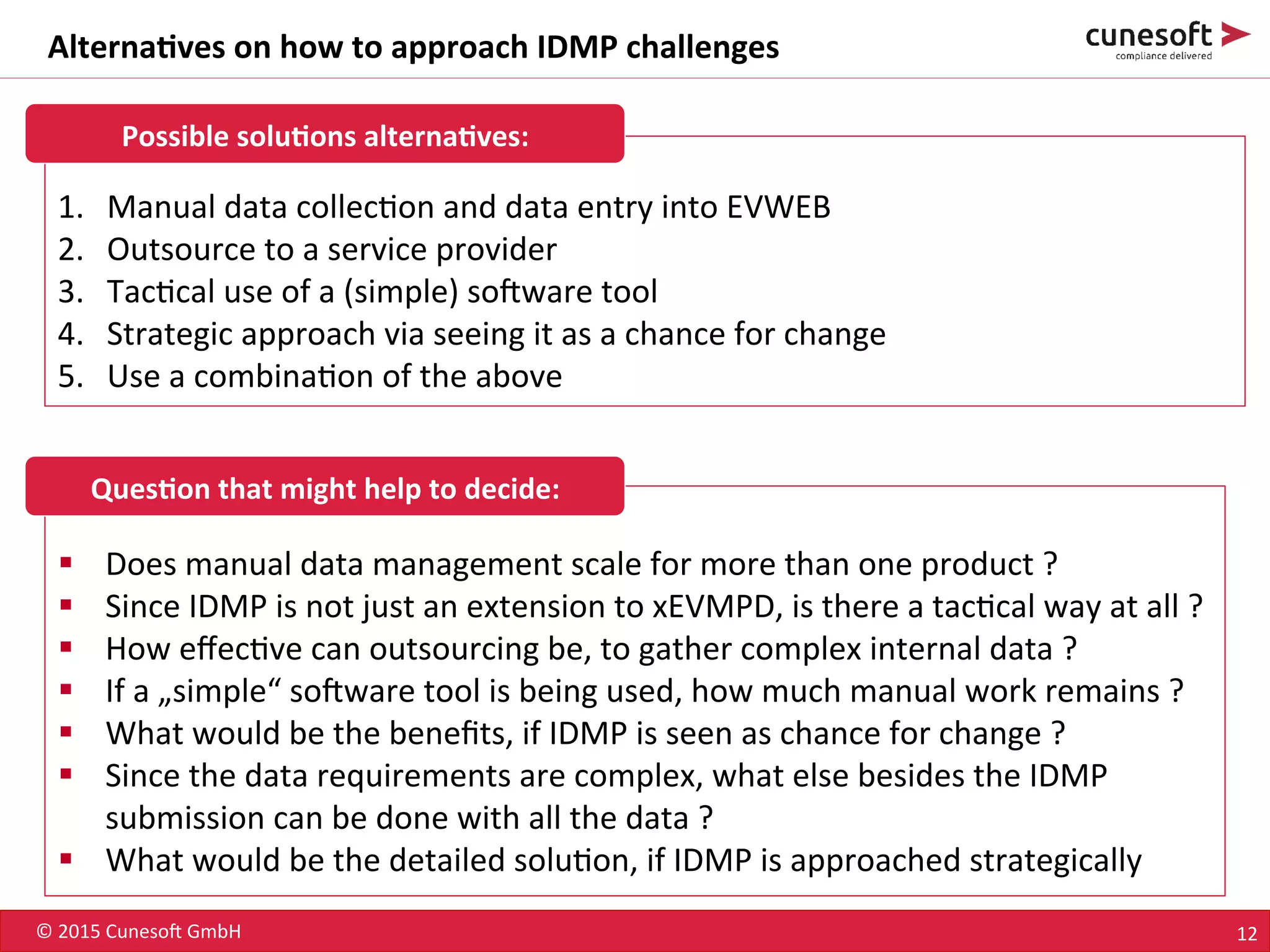
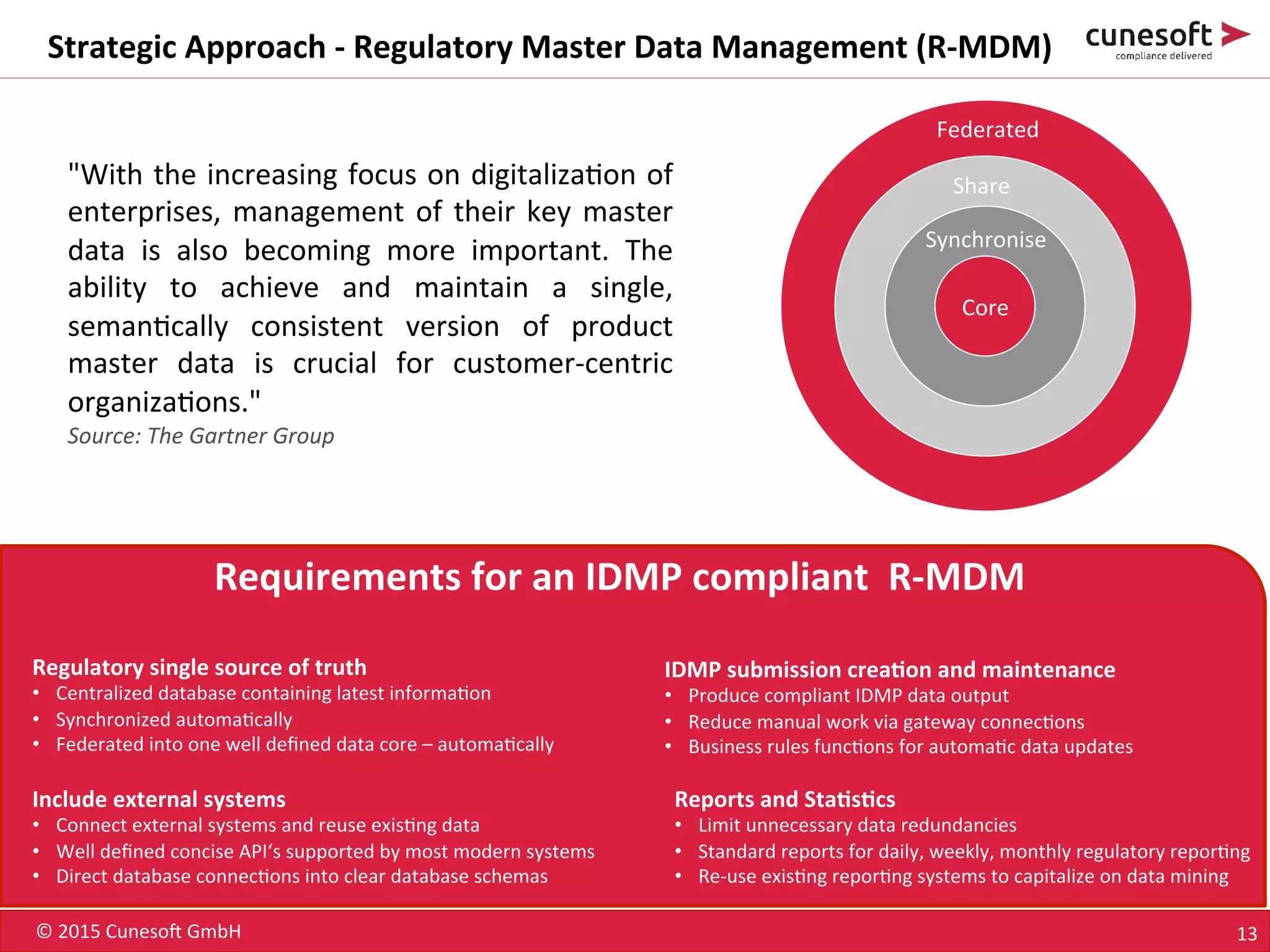
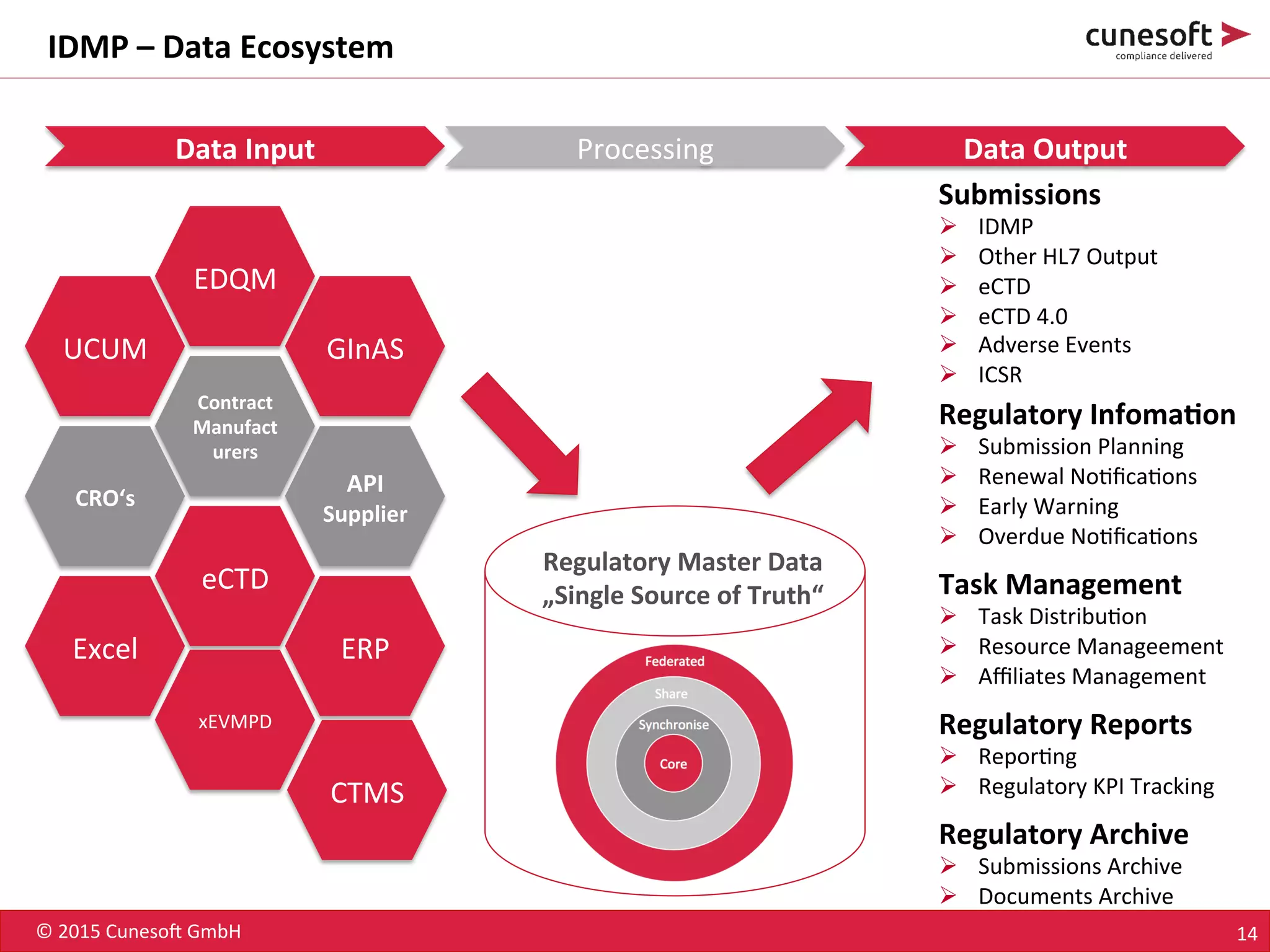
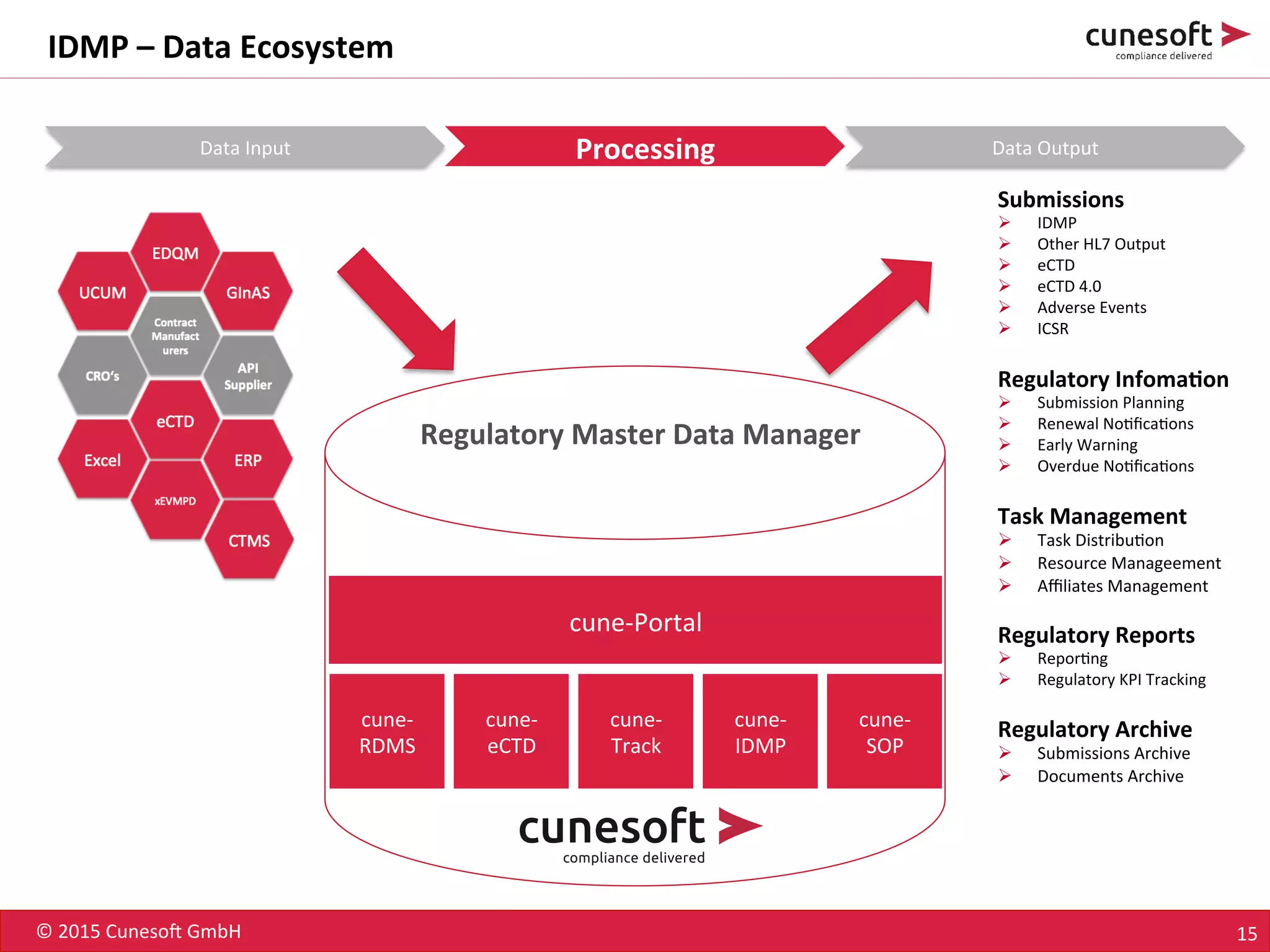
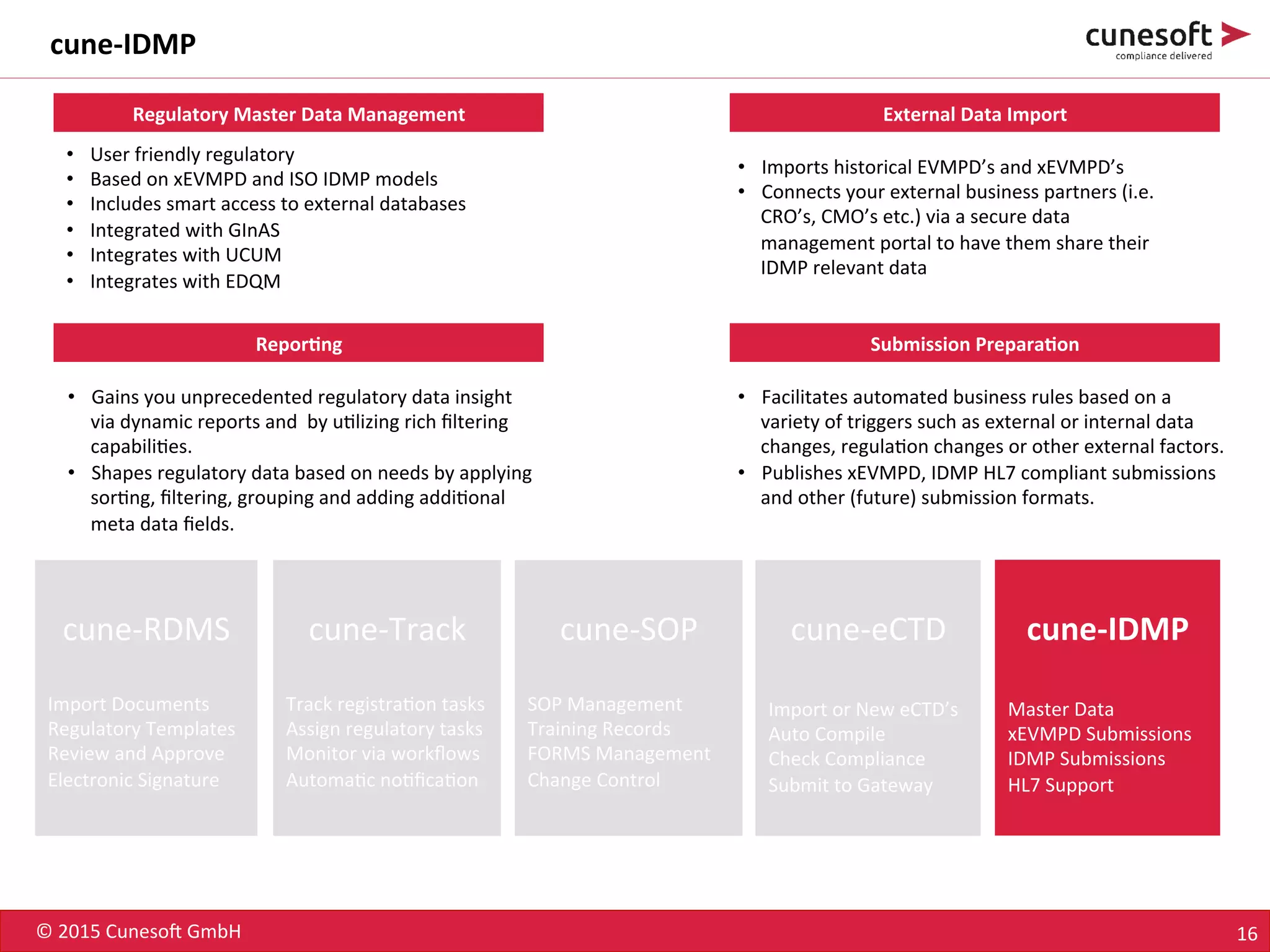
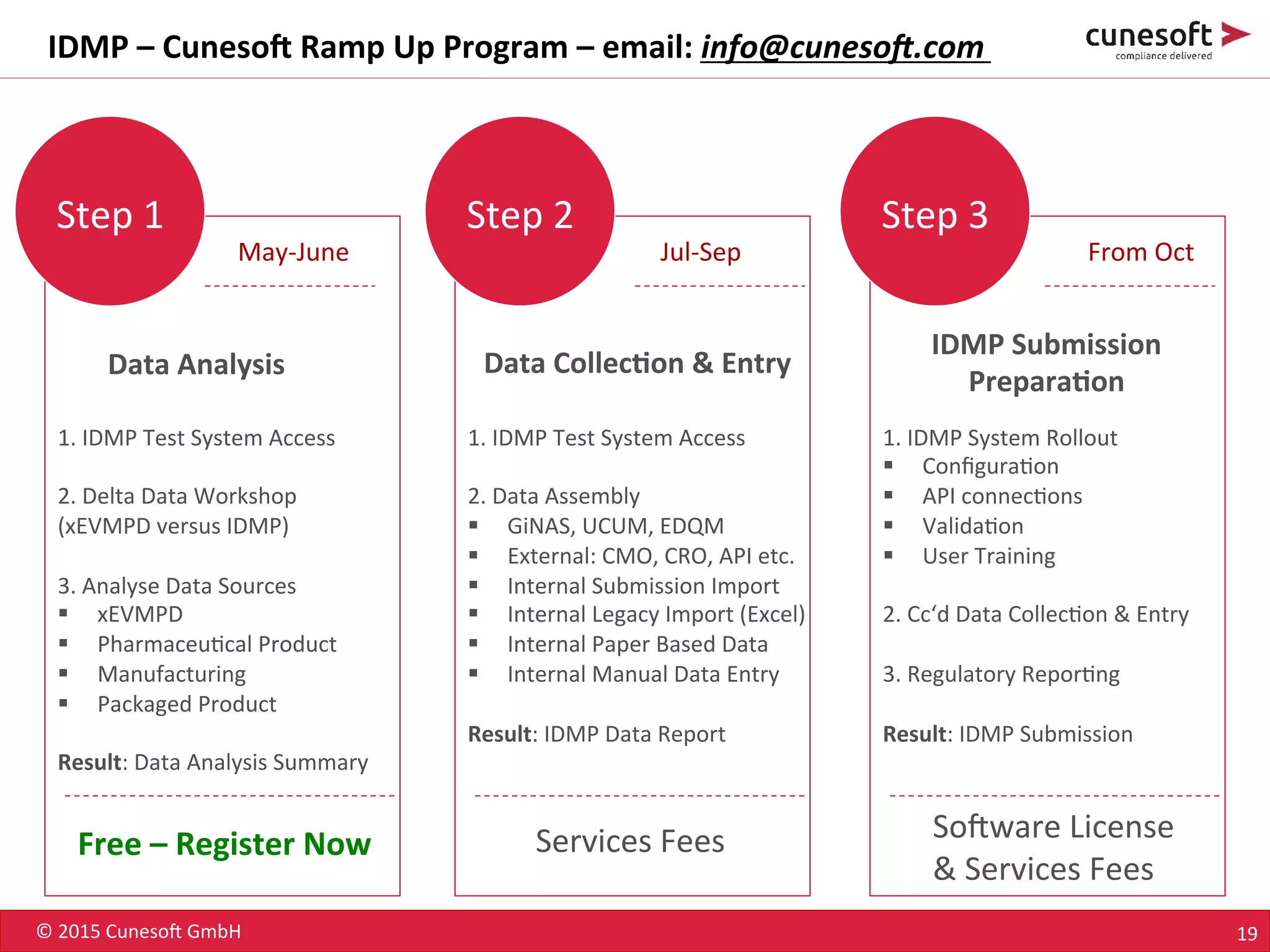
The document outlines the identification of medicinal products (IDMP) and the benefits of a master data-driven approach, detailing Cuneso GmbH's regulatory software solutions and project methodologies. It highlights the complexities of IDMP implementation, the required data management, and the company's ramp-up program aimed at facilitating compliance by using integrated software tools. Additionally, it emphasizes the necessity of centralized master data management to ensure regulatory compliance and streamline submissions.