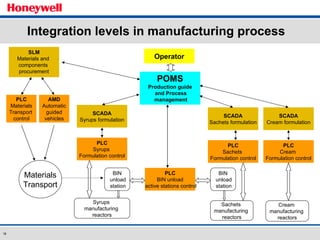
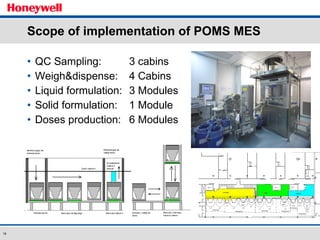
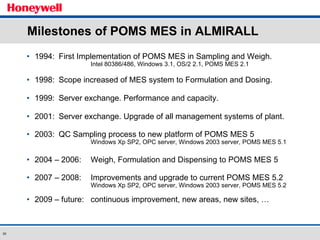
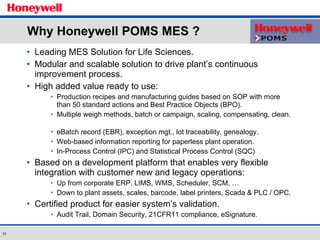
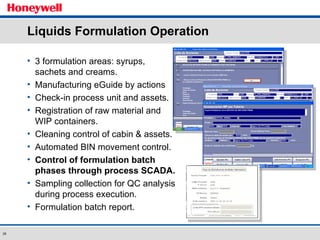
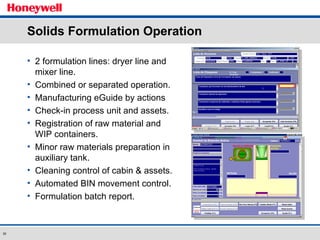
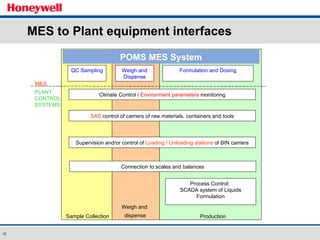
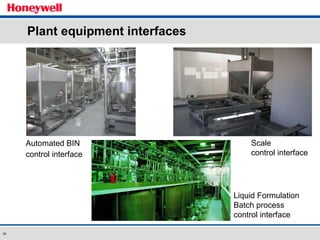
The document discusses the implementation of a POMS MES system at Almirall, a pharmaceutical company. It describes how the MES system improved quality and production operations through integrated management of processes like purchasing, manufacturing, quality control and logistics. Key benefits included improved traceability, compliance and flexibility to remain competitive through continuous optimization of manufacturing execution.





















![Imagination is what makes all things possible! Thank You, for more information please contact us Xavier Raso [email_address] David Badia [email_address] www.almirall.com www.asesa.net](https://image.slidesharecdn.com/2009hugasesaalmirall-110414170248-phpapp02/85/Honeywell-User-s-Group-Almirall-s-MES-case-study-39-320.jpg)