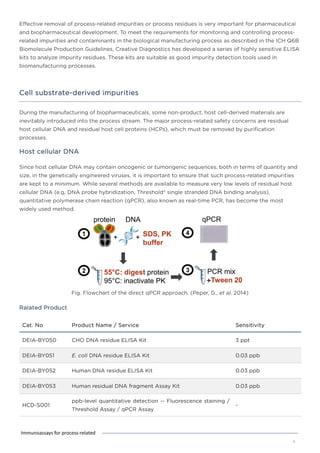
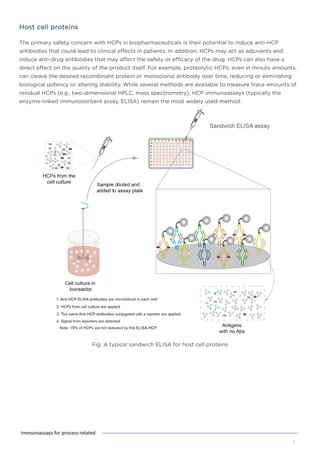
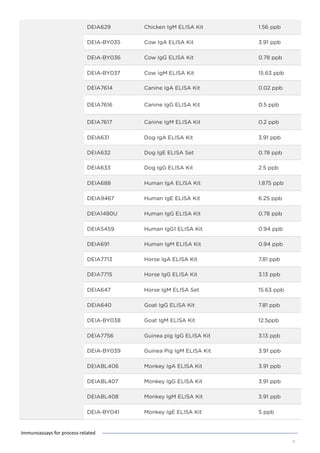
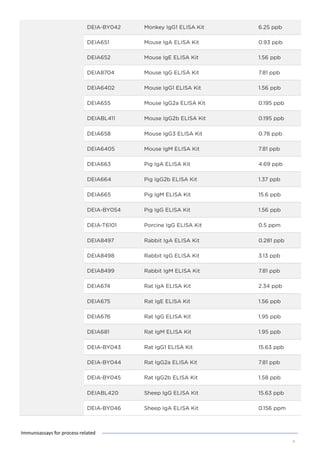
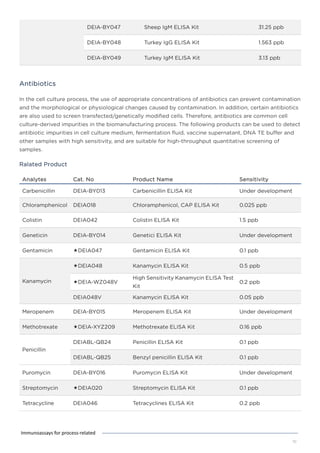
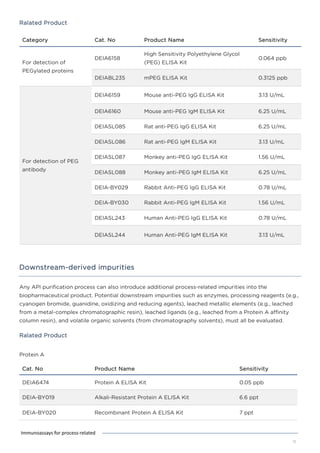
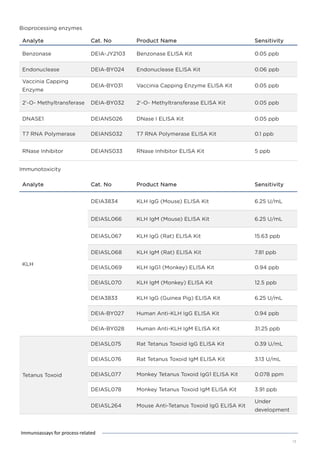
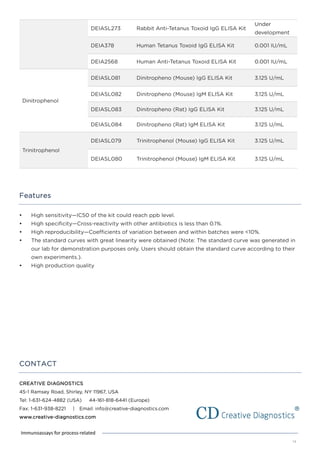
This document discusses various process-related impurities that can occur during the production of biologics and immunoassays to detect them. It describes three categories of impurities: cell substrate-derived (e.g. host cell DNA, proteins), cell culture-derived (e.g. media components, antibiotics), and downstream-derived (e.g. processing reagents). It provides details on specific impurities like host cell DNA, proteins, and culture media components, as well as ELISA kits available to detect residual levels of these impurities.