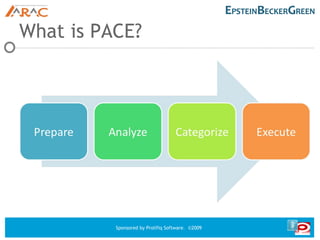
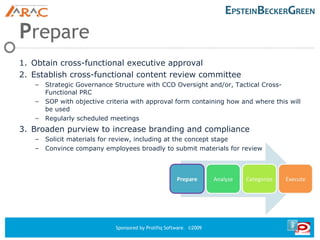
This document discusses PACE, a collaborative process for preparing, analyzing, categorizing, and executing materials for regulatory compliance. PACE involves obtaining executive approval, establishing a content review committee, analyzing claims and guidelines, categorizing materials, educating employees, and using technology for controlled dissemination of materials. The goal of PACE is to provide traceability and accountability for materials through an efficient review process that considers regulations and avoids compliance issues. Questions about PACE can be directed to the speakers.







![Questions & Answers Elsa Chi Abruzzo, President, ARAC [email_address] Steven Skwara, Partner, EBG [email_address] Sponsored by: Maureen Shaffer, VP Life Sciences, Prolifiq [email_address]](https://image.slidesharecdn.com/navigatingfinal-elsa-091014103310-phpapp02/85/PACE-A-Collaborative-Process-Elsa-Chi-Abruzzo-President-ARAC-LLC-10-320.jpg)
