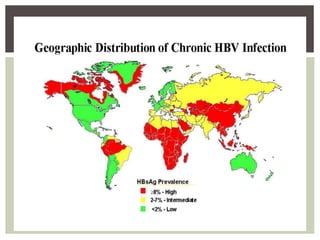
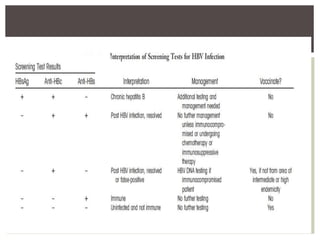
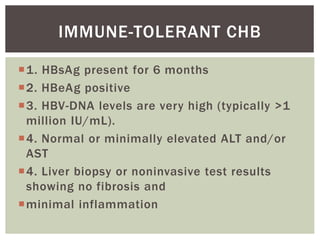
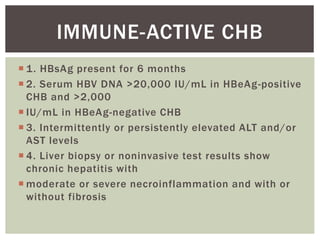
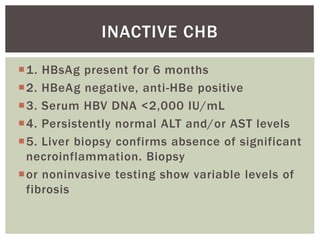
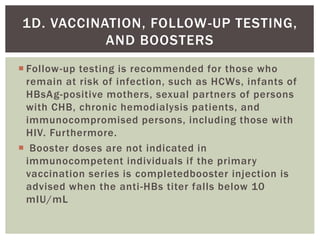
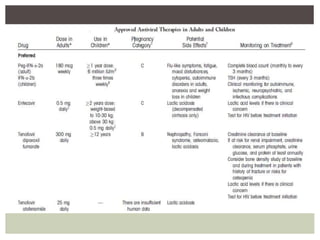
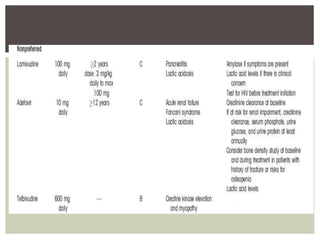
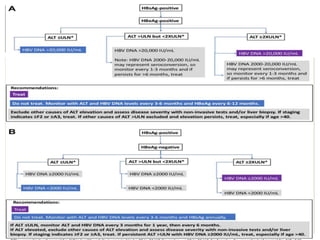
This document discusses Hepatitis B virus (HBV) infection. It notes that over 2 billion people have been infected with HBV worldwide, with 360 million having chronic infections. HBV infection has a worldwide distribution and is endemic in many parts of Africa, Asia, and the Middle East. Groups at high risk for HBV infection who should be screened include people born in highly endemic regions, healthcare workers, injection drug users, men who have sex with men, and household contacts of HBV-infected individuals. Chronic HBV infection can be diagnosed based on the presence of HBsAg for over 6 months and is classified into immune tolerant, immune active, and inactive phases based on viral load and liver enzyme levels. Vaccination against