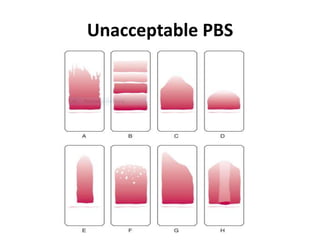
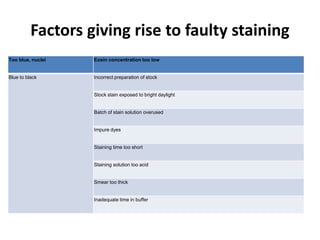
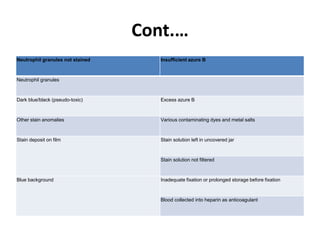
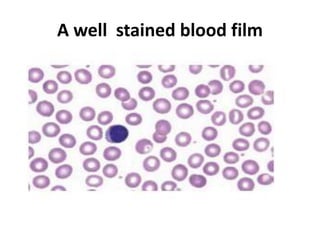
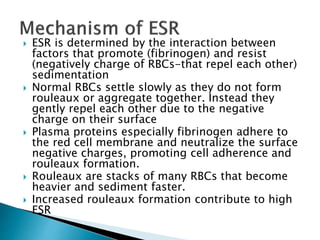
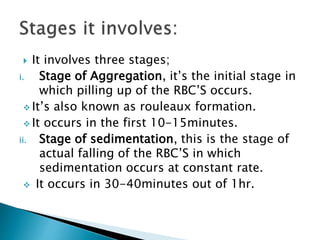
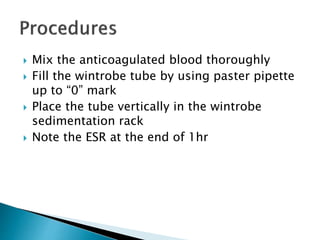
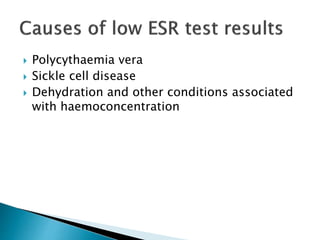
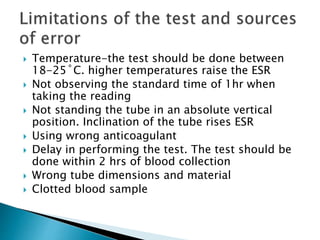
This document provides information on how to perform an erythrocyte sedimentation rate (ESR) test. It begins by explaining that ESR measures the rate at which red blood cells sediment in one hour, and though nonspecific, an increased ESR can indicate infection, inflammation or malignancy. It then notes some limitations before describing the basic principles behind ESR, including how plasma proteins promote rouleaux formation and faster sedimentation. Finally, it states that the Westergren method is the preferred technique for determining ESR over the Wintrobe method.