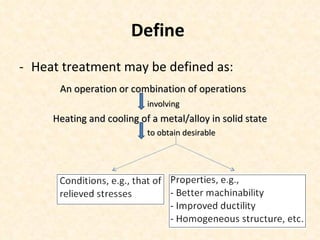
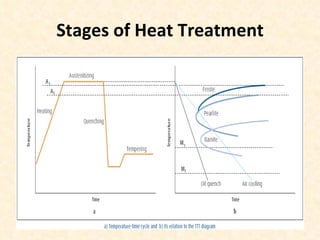
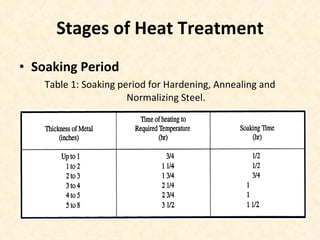
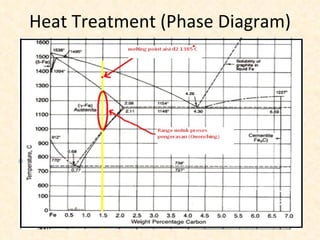
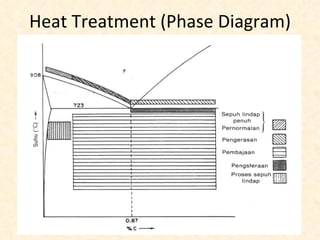
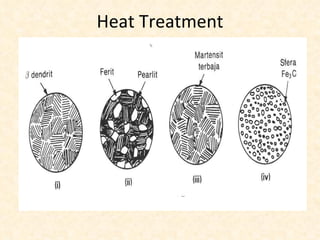
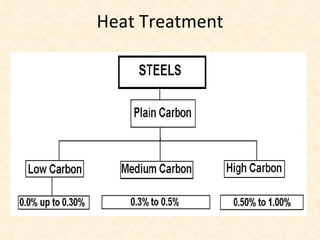
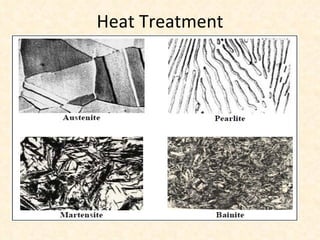
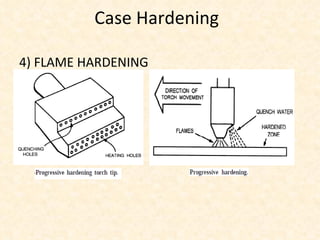
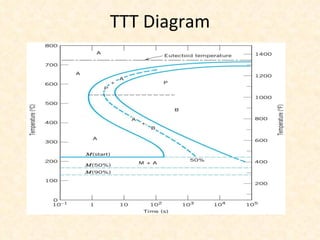
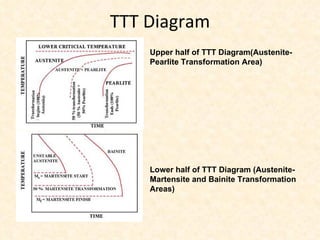
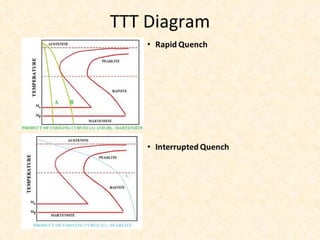
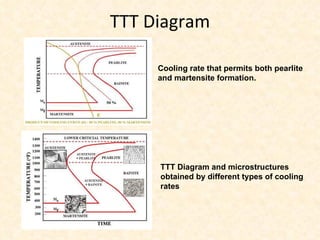
The document provides an outline on heat treatment processes. It defines heat treatment and its purposes, discusses heat treatment theory and the stages of heat treatment including heating, soaking, and cooling. It describes various heat treatment processes like annealing, normalizing, hardening, and tempering. It also discusses case hardening techniques like carburizing, cyaniding, and nitriding. Finally, it introduces the TTT diagram and the microstructures obtained from different cooling rates.