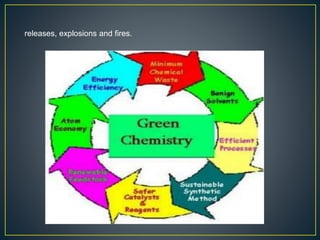
Green chemistry is a philosophy that encourages designing chemical products and processes to minimize hazardous substances and environmental impact. It aims to reduce risks to human health and eliminate pollution. The 12 Principles of Green Chemistry developed in 1991 provide a framework, including preventing waste, designing safer chemicals that break down easily, and using renewable, non-toxic resources whenever possible. Examples include medicines with fewer side effects, biodegradable plastics, and low-VOC paints.