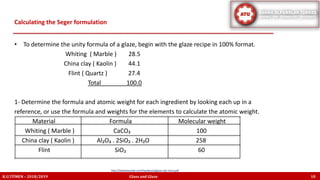
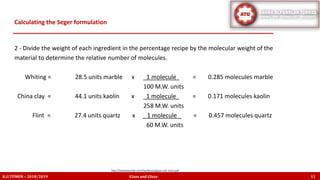
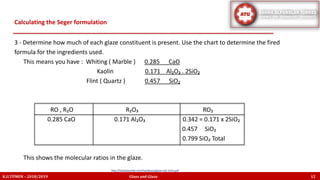
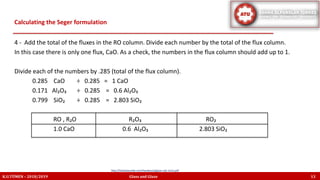
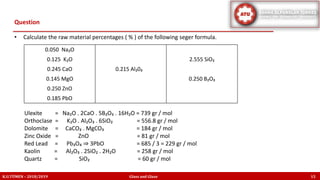
The document discusses the Seger formula used for evaluating glaze recipes, which organizes glaze components by oxide composition instead of raw materials. It explains the categorization of oxides into fluxes, stabilizers, and glass formers, highlighting their roles in glaze behavior and characteristics. Additionally, it provides insights on calculating the unity formula for glazes, which represents the ratio of ceramic oxides, and emphasizes the importance of understanding oxide ratios for effective glaze formulation.