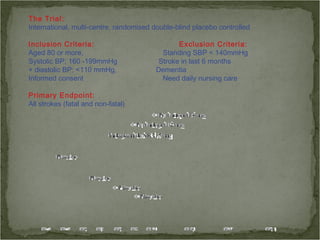
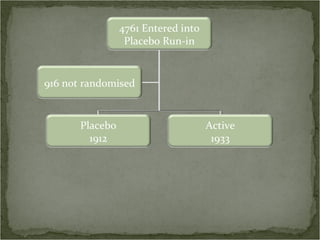
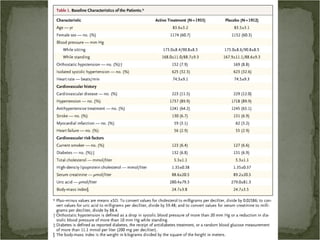
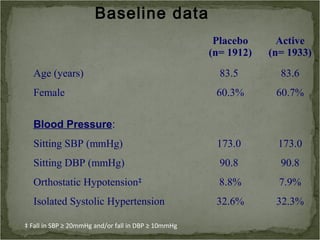
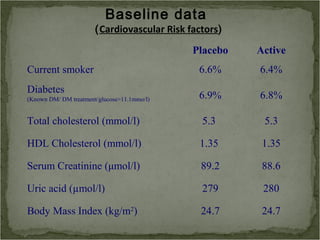
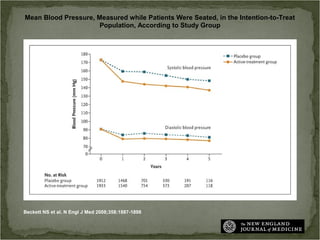
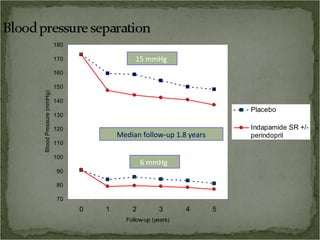
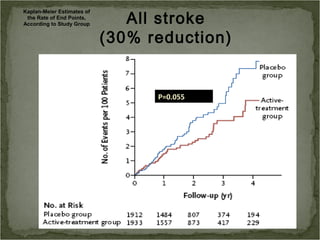
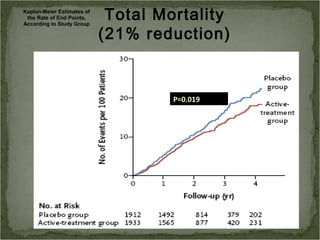
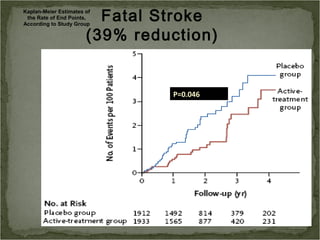
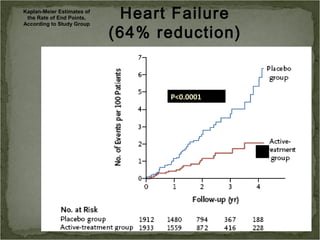
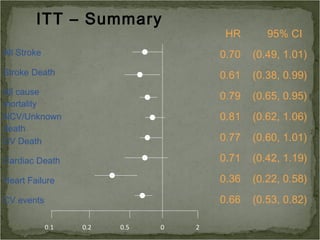
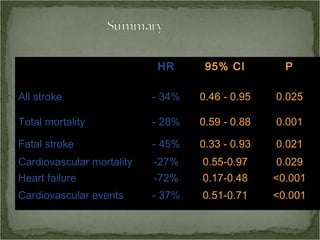
Active treatment of isolated systolic hypertension in elderly patients using indapamide with or without perindopril resulted in significant reductions in mortality, stroke, and heart failure compared to placebo. Systolic blood pressure was reduced by 15 mmHg in the active treatment group. The benefits of treatment were seen early with number needed to treat of 94 for stroke and 40 for mortality reduction over 2 years. Treatment was also found to be safe with no significant differences in adverse lab values between groups.












![Primary outcome:
Combined fatal CHD or non-
fatal myocardial infarction,
analyzed by intent-to-treat
Secondary outcomes:
ll- cause mortality,
troke,
ombined CHD (primary outcome, coronary revascularization, or angina with hospitalization)
ombined CVD (combined CHD, stroke, treated angina without hospitalization,
heart failure [HF],
peripheral arterial disease](https://image.slidesharecdn.com/geripres-121115133459-phpapp01/85/Geri-pres-30-320.jpg)





