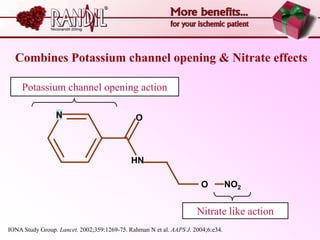
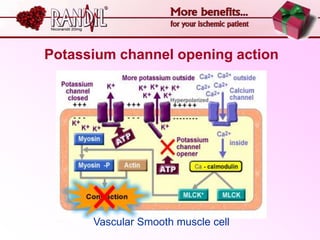
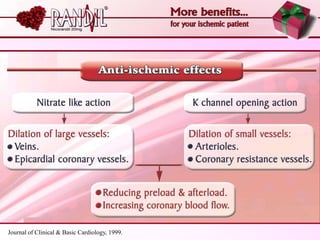
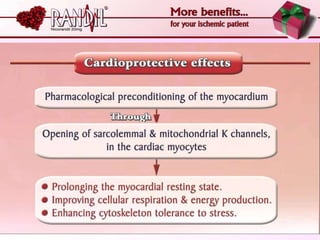
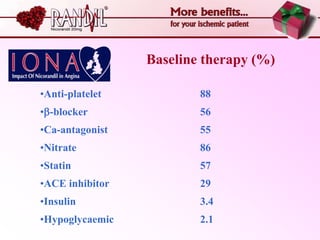
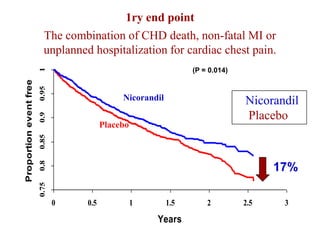
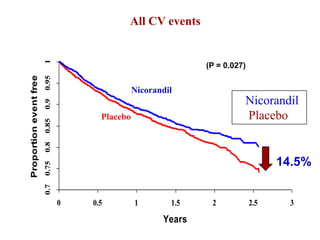
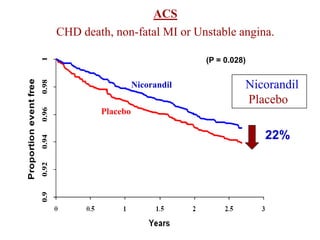
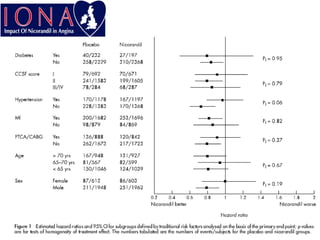
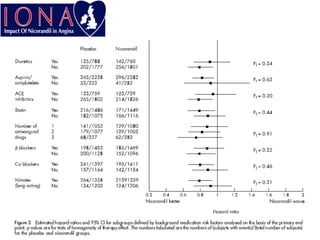
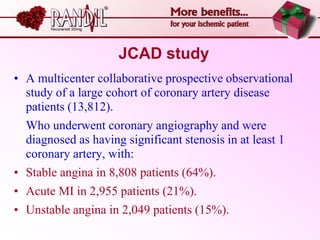
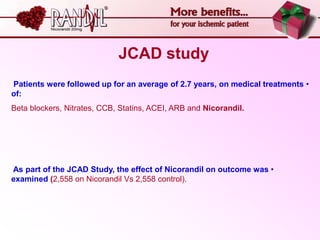
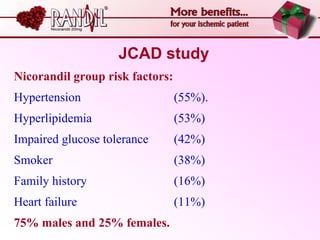
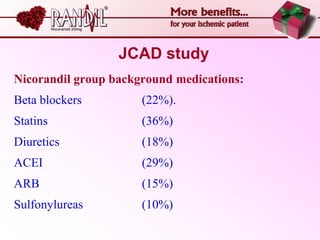
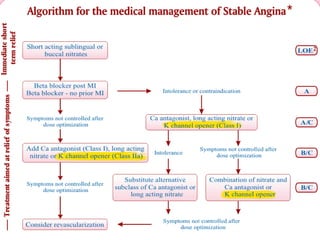
- A study compared the effects of glibenclamide and gliclazide, two sulphonylureas, on ischemic preconditioning (IPC) and nicorandil-induced myocardial protection in rats.
- Glibenclamide abolished the protective effects of both IPC and nicorandil, but gliclazide did not affect IPC or nicorandil-induced protection.
- Nicorandil caused a partial depolarization of mitochondrial membrane potential, an effect blocked by glibenclamide but not gliclazide. These results suggest gliclazide may allow protective effects in diabetic patients at risk of acute coronary syndromes unlike