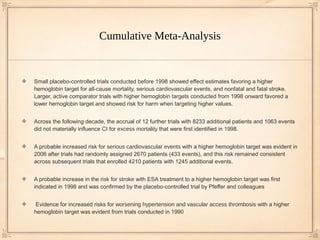
1) A meta-analysis of 27 randomized trials found that treating anemia in chronic kidney disease patients with erythropoiesis-stimulating agents (ESAs) to achieve higher hemoglobin levels increased risks of stroke, worsening hypertension, and vascular access thrombosis compared to lower hemoglobin targets.
2) No significant differences were found between higher and lower hemoglobin targets for risks of death, cardiovascular events, or progression to kidney failure requiring dialysis.
3) While higher hemoglobin targets reduced need for blood transfusions, they increased use of intravenous iron therapy.







![Meta-Analysis
Risks for fatal and nonfatal stroke, vascular access thrombosis, and worsening hypertension
were increased with a higher hemoglobin target than with a lower hemoglobin target
Patients randomly assigned to a higher hemoglobin target were less likely to require a blood
transfusion (8 trials; 6482 patients) (RR, 0.61 [95% CI, 0.49 to 0.77]) but were more likely to
receive intravenous iron therapy (6 trials; 2283 patients) (RR, 1.57 [CI, 1.13 to 2.20])
No statistically significant difference in the risk for all-cause mortality, serious cardiovascular
events, or fatal and nonfatal myocardial infarction between a higher hemoglobin target and a
lower target
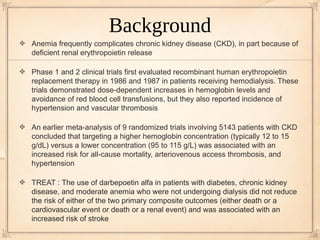
In the 10 studies conducted in people with CKD who were not dialysis-dependent, the risk for
end-stage kidney disease requiring renal replacement therapy did not statistically significantly
differ when hemoglobin target groups were compared
No statistically significant differences between treatment groups in left ventricular mass at the
end of treatment (4 trials; 1544 patients; mean difference, 0.14 g/m2 [CI, −4.60 to 4.88 g/m2])](https://image.slidesharecdn.com/journalclub72010-121115134234-phpapp02/85/Journal-club-7-2010-11-320.jpg)